New publications
Find here the further new publications:
<><><>
https://www.researchgate.net/p
<><><>
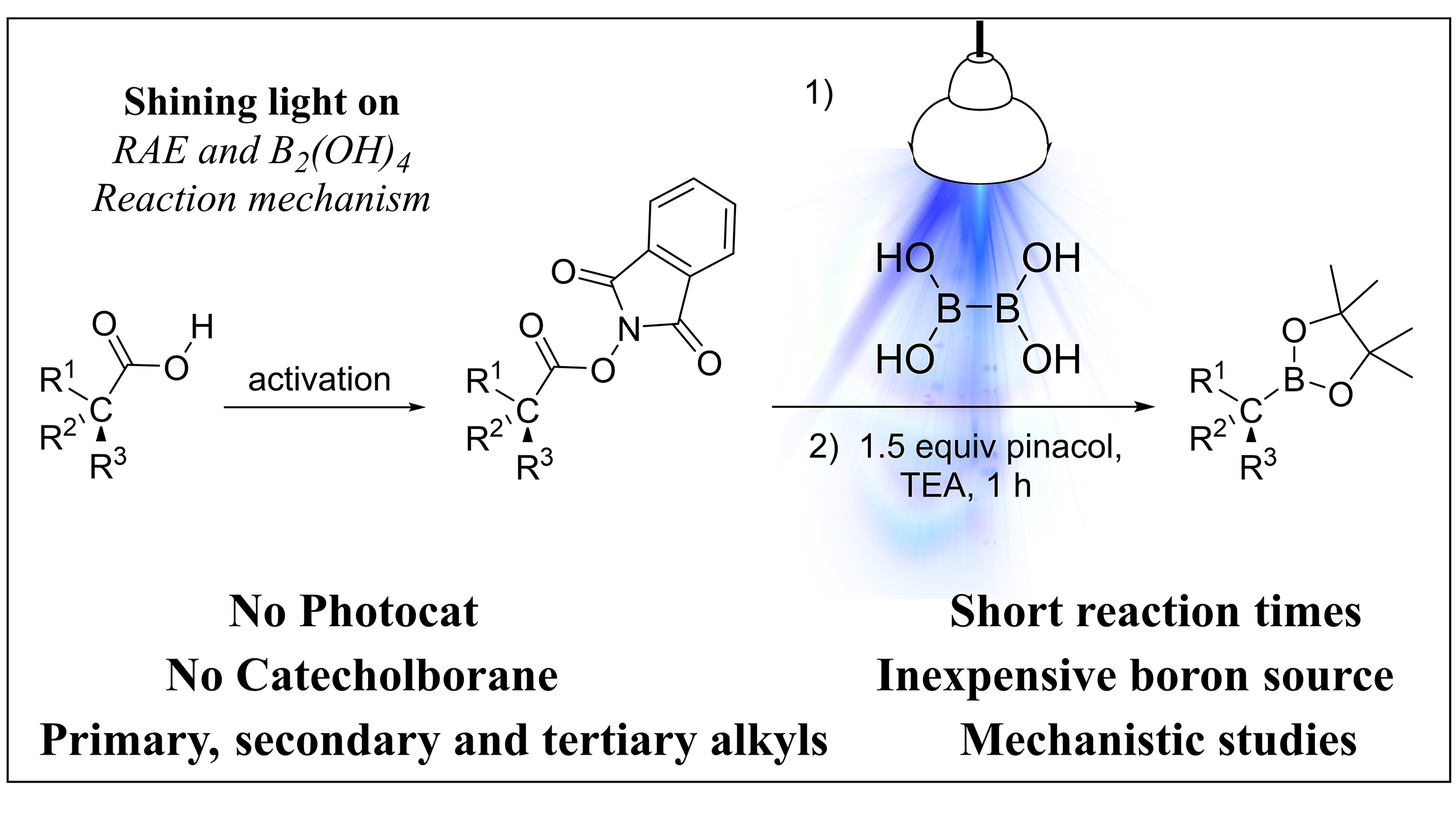
93. Photoinduced Decarboxylative Borylation of N‑Hydroxyphthalimide Esters with Hypoboric Acid
Photoinduced Decarboxylative Borylation of N‑Hydroxyphthalimide Esters with Hypoboric Acid, Bálint Nagy, Zsombor Gonda, Tamás Földesi, Péter Pál Fehér, András Stirling, Gergely L. Tolnai, Zoltán Novák, Org. Lett. 2024, 26, 2292–2296. DOI: 10.1021/acs.orglett.4c00511 | [Full Text Link] [Supp. Info Link]
We developed a visible-light driven photochemical transformation, in which activated primary, secondary and tertiary alkylcarboxylic acids were converted into the corresponding boronic esters in the absence of catechol and any added photocatalyst. The procedure relies on the utilization of hypoboric acid and redox-active esters of alkylcarboxylic acids, ensuring simple and economic procedure. Quantum chemical calculations and mechanistic considerations provide deeper insights into the mechanism of photochemical borylation reactions.
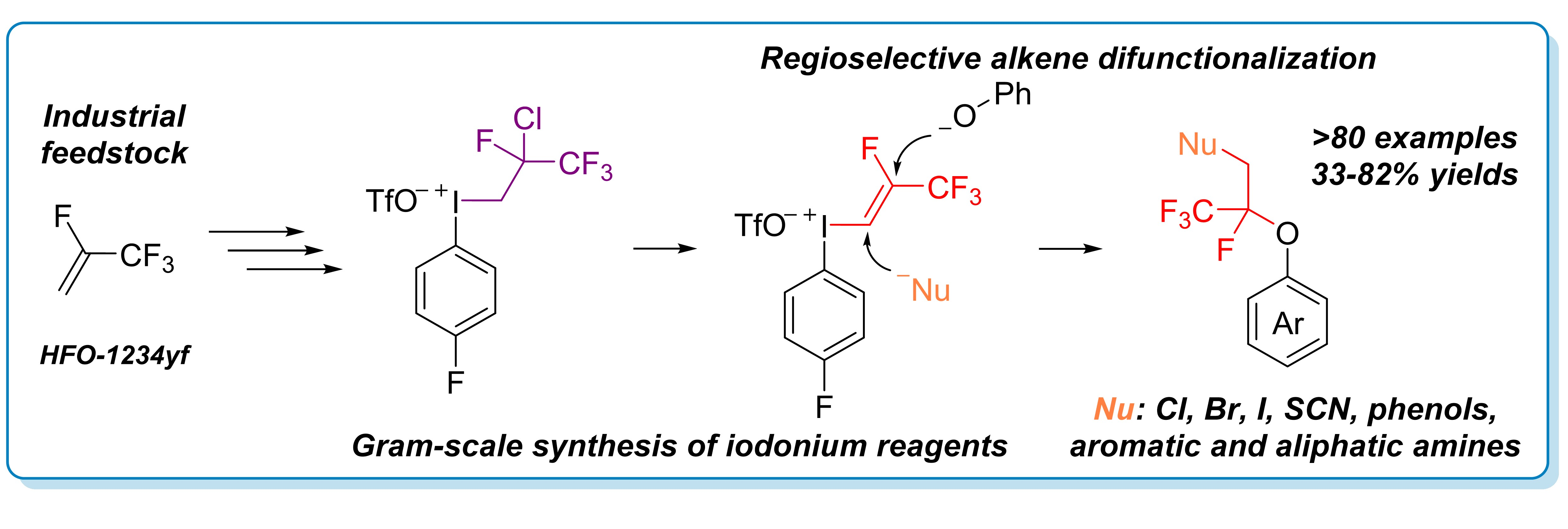
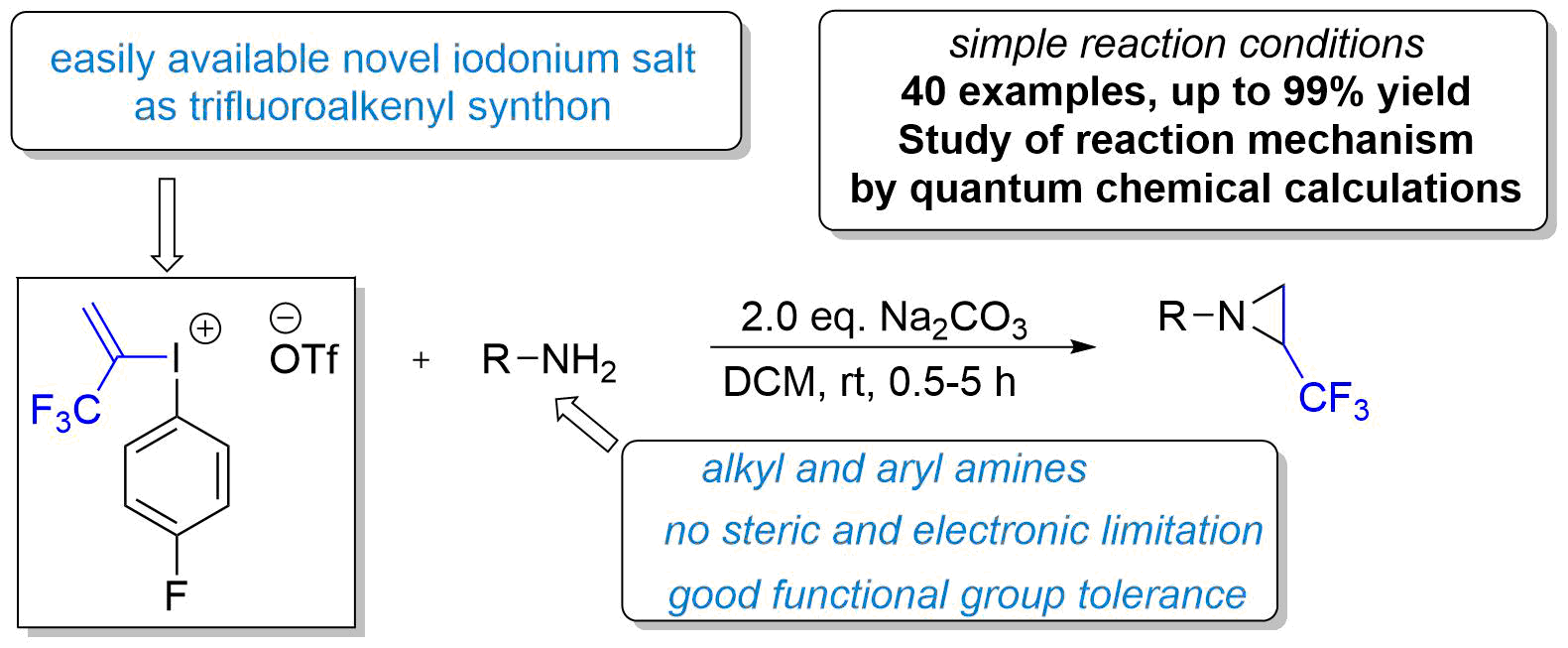
92. Iodonium based regioselective double nucleophilic alkene functionalization of a hydrofluoroolefin scaffold
Iodonium based regioselective double nucleophilic alkene functionalization of a hydrofluoroolefin scaffold, János T. Csenki, Zoltán Novák, Chem. Commun., 2024, 60, 726-729. DOI: 10.1039/D3CC04985J | [Full Text Link] [Supp. Info Link]
Herein, we report a modular regioselective alkene difunctionalization strategy based on the use of hydrofluoroolefin (HFO) gas as fluorous feedstock material. The transformation of the HFO gas to iodonium salt creates vicinal electrophilic sites readily available for a broad range of nucleophiles.
91. Fluoroalkylations and Fluoroalkenylations with Iodonium Salts
Fluoroalkylations and Fluoroalkenylations with Iodonium Salts, Ferenc Béke, János T. Csenki, Zoltán Novák, Chem. Rec. 2023, 23, e202300083. DOI: 10.1002/tcr.202300083 | [Full Text Link]
Synthesis and applications of fluoroalkyl and fluoroalkenyliodonium salts are summarized in this account article, focusing preferably to the reagents designed in our laboratory in the last decade. Among these reagents trifluoroethyl(aryl)iodonium salts have been used most frequently to build carbon-carbon and carbon-heteroatom bonds in simple nucleophilic substitutions and through transition metal catalyzed coupling reactions. Iodonium salts equipped with unsaturated fluorinated function showed diverse reactivity due to their electron deficient character, and these molecular motifs enable cycloadditions and nucleophilic additions to prepare fluorinated carbo- and heterocyclic molecules. Beyond the overview of existing transformations, with the presented collection, we aim to inspire future developments of iodonium reagents and their application in organic synthesis.
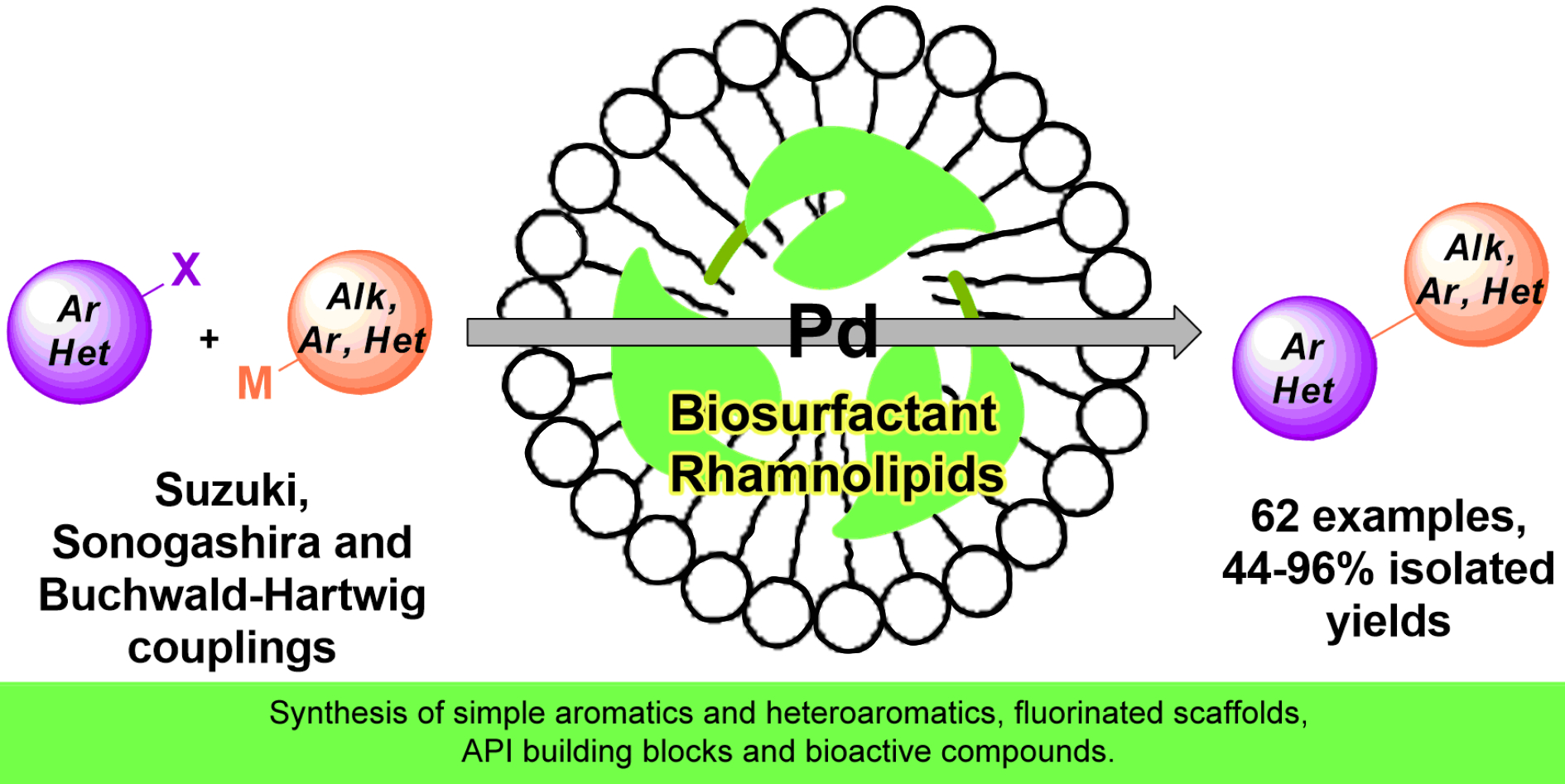
90. Gate to Parallel Universe: Utilization of Biosurfactants in Micellar Catalysis
Gate to Parallel Universe: Utilization of Biosurfactants in Micellar Catalysis, Réka Adamik, Attila R. Herczegh, Imre Varga, Zoltán May, Zoltán Novák, Green Chem.. 2023, ASAP. DOI: 10.1039/D3GC00365E | [Full Text Link] [Supp. Info Link]
Micellar catalysis offers a green alternative media for catalytic transformations by solubilization of hydrophobic organic compounds. Biosurfactants represent a sustainable alternative to synthetic surfactants due to their biodegradability, low toxicity, and production from renewable feedstocks. In this work, we demonstrated the applicability of biosurfactant rhamnolipids in various micellar cross couplings, and prepared special fluorinated conjugated systems, Active Pharmaceutical Ingredient (API) building blocks and bioactive compounds
89. Steric Effects in ortho C-H Activation of Aromatic Systems
Steric Effects in ortho C-H Activation of Aromatic Systems, Z. Novák, B. L. Tóth, A. Stirling, Handbook of C-H functionalization. Chapter: Role of Directing Groups. 2022, Wiley. DOI: 10.1002/9783527834242.chf0080 | [Full Text Link] [Table of Content of The Book]
Transition metal-catalyzed directed aromatic ortho C-H activation offers many possibilities for the functionalization of various substrates having different directing groups. However, the reactivity of the substrate is determined by electronic and steric factors. Herein, we present and discuss a recently developed model which considers only the steric properties of the substrates to assess their reactivity in ortho C-H activation in the presence of ortho substituents. Dihedral angle between the directing group and the aromatic ring influences the coordination and the C-H activation elementary step, thus determine reactivities and the stability of metal-complex intermediates. The presentation of the theoretical model is followed by several literature examples from substrates having borderline reactivities, to explore and describe the most interesting region of reactivity. The most commonly used directing groups are discussed taking into consideration their key structural elements, and reactivity trends are presented with the help of complex formation and reactions of each substrate group. The lack of transformation is also discussed by showing representatives of unreactive substrates.

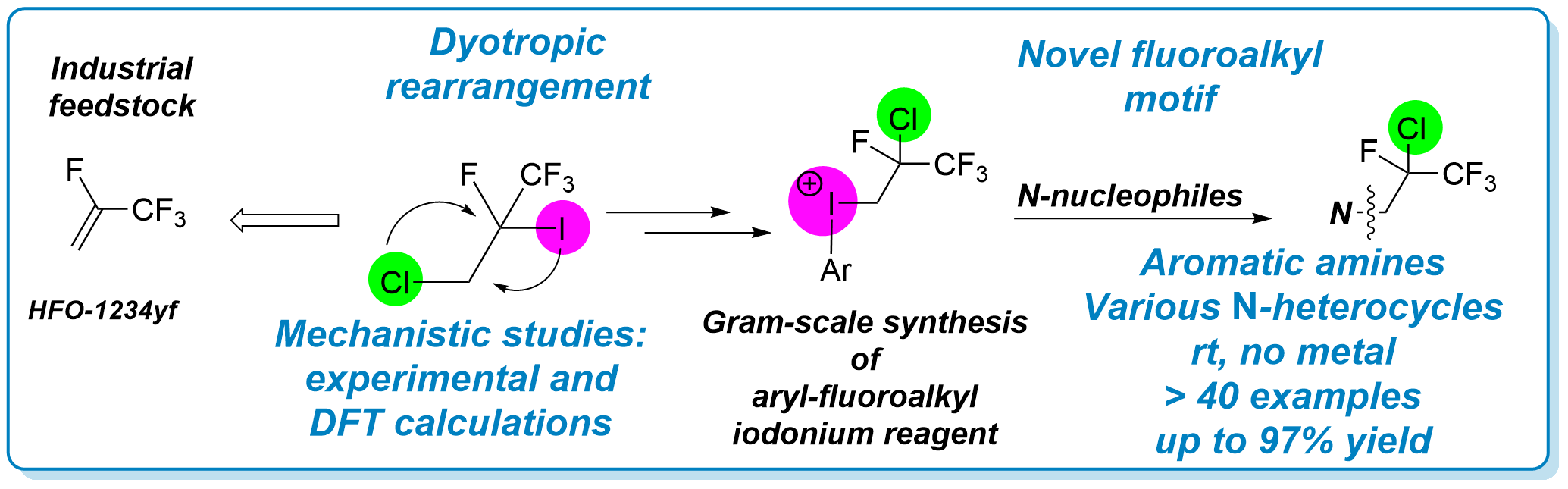
88. Synthesis of Hydrofluoroolefin-Based Iodonium Reagent via Dyotropic Rearrangement and Its Utilization in Fluoroalkylation
Synthesis of Hydrofluoroolefin-Based Iodonium Reagent via Dyotropic Rearrangement and Its Utilization in Fluoroalkylation, János T. Csenki, Balázs L. Tóth, Ferenc Béke, Bálint Varga, Péter P. Fehér, András Stirling, Zsuzsanna Czégény, Attila Bényei, Zoltán Novák, Angew. Chem. Int. Ed. 2022, 61, ASAP. DOI: 10.1002/anie.202208420 | [Full Text Link] [Supp. Info Link] [Crystal Data (CIF)]
The synthesis of a novel hypervalent iodonium salt was developed with the exploitation of a dyotropic rearrangement of iodine and chlorine functions in fluoroalkyl chains from HFO-1234yf gas. The electrophilic reagent enabled the direct and metal-free introduction of a special fluoroalkyl group into aniline derivatives and N-containing heteroaromatic compounds. Detailed experimental and computational data were collected and used to propose a mechanistic description of this unique rearrangement.
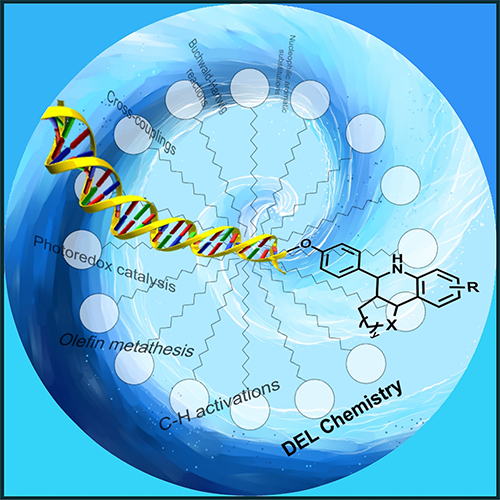
87. The Potential of Micellar Media in the Synthesis of DNA-Encoded Libraries
The Potential of Micellar Media in the Synthesis of DNA-Encoded Libraries, Réka Adamik, Balázs Buchholcz, Ferenc Darvas, Gellért Sipos, Zoltán Novák, Chem. Eur. J. 2022, 28, e202103967. DOI: 10.1002/chem.202103967 | [Full Text Link]
DNA-encoded libraries (DELs) have become a widely used technology in drug discovery. The use of micelle-forming surfactants provides new possibilities for synthetic organic chemistry and could be a promising technique for DNA-compatible chemistry as well. This review provides a summary of existing examples of micellar DEL approaches, together with a selection of micellar organic transformations fundamentally suitable for DEL synthesis.
Highlighted as a Frontispiece. Chem. Eur. J. 2022, 28, e202282062. DOI: 10.1002/chem.202282062 | [Full Text Link]
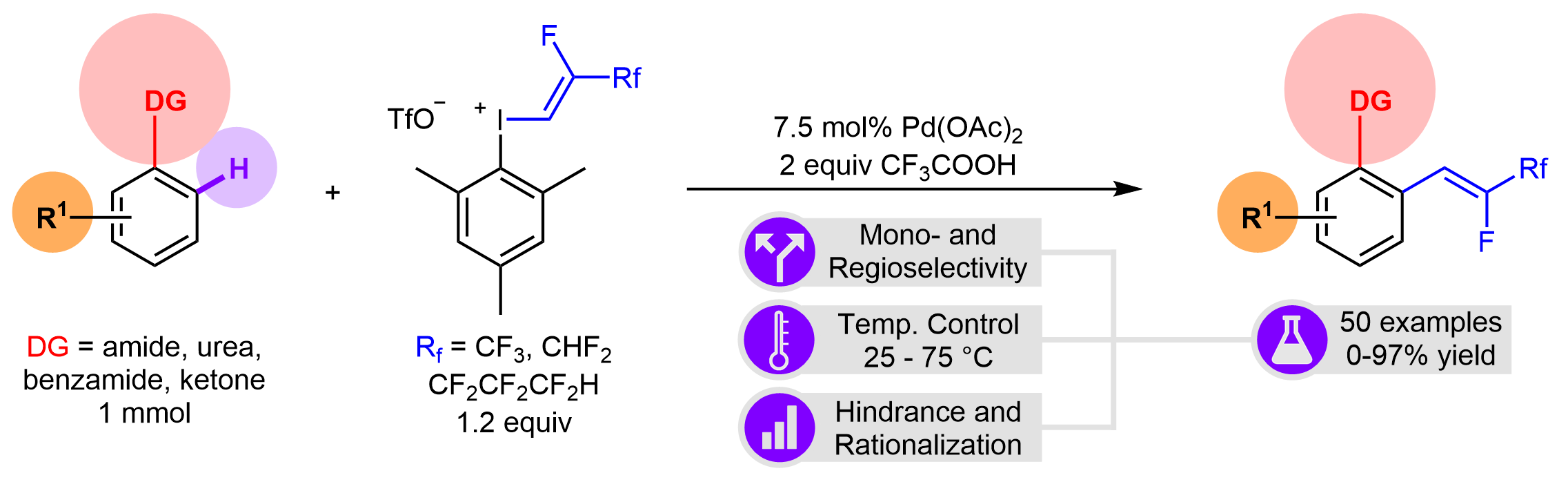
86. Z-Selective Fluoroalkenylation of (Hetero)Aromatic Systems by Iodonium Reagents in Palladium-Catalyzed Directed C-H Activation
Z-Selective Fluoroalkenylation of (Hetero)Aromatic Systems by Iodonium Reagents in Palladium-Catalyzed Directed C-H Activation, Balázs L. Tóth, Gergő Sályi, Attila Domján, Orsolya Egyed, Attila Bényei, Zsombor Gonda, Zoltán Novák, Adv. Synth. Catal. 2022, 364, 348-354. DOI: 10.1002/adsc.202101108 | [Full Text Link] [Supp. Info. Link]
The direct and catalytic incorporation of fluorine containing molecular motifs into organic compounds resulting high-value added chemicals represents a rapidly evolving part of synthetic methodologies, thus this area is in the focus of pharmaceutical and agrochemical research. Herein we report a stereoselective procedure for direct fluorovinylation of aromatic and heteroaromatic scaffolds. This methodology development has been realized by palladium-catalyzed ortho C-H activation reaction of aniline derivatives featuring the regioselectivity via directing groups such as secondary of tertiary amides, ureas or ketones. The application of non-symmetrical aryl(fluoroalkenyl)-iodonium salts as fluoroalkenylating agents allowed mild reaction conditions in general for this transformation. The scope and limitations have been thoroughly investigated and the feasibility has been demonstrated by more than 50 examples.
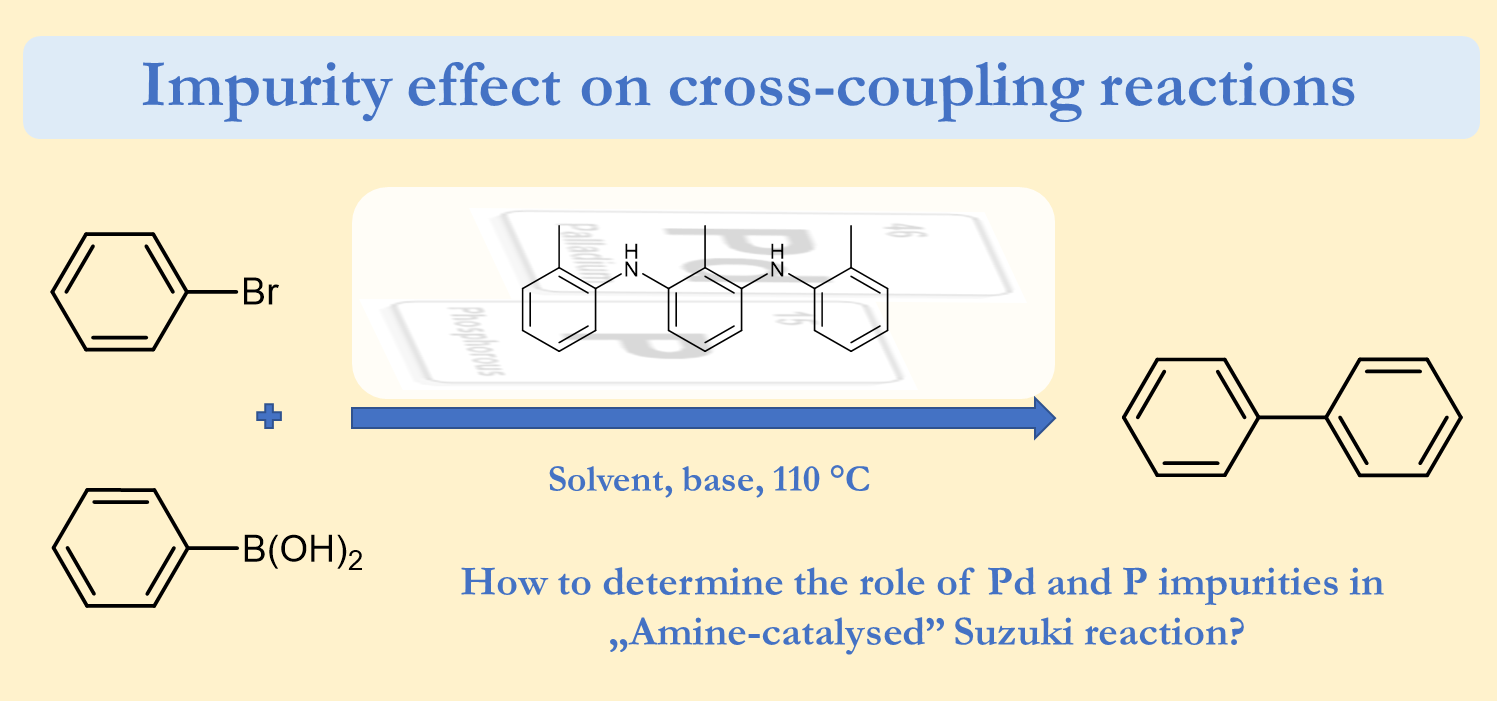
85. Revisiting the amine-catalysed cross-coupling
Revisiting the amine-catalysed cross-coupling, Zoltán Novák, Réka Adamik, János T. Csenki, Ferenc Béke, Regina Gavaldik, Bálint Varga, Bálint Nagy, Zoltán May, János Daru, Zsombor Gonda, Gergely L. Tolnai, Nature Catal. 2021, 4, 991–993. DOI: 10.1038/s41929-021-00709-8 [Supp. Info. Link] [Supp. Info. Link 2] [ChemRxiv PrePrint]
Several efforts have been made for the replacement of noble metal palladium in cross-coupling reactions, maintaining high efficiency of the target transformation. In several cases it is possible to perform the chemistry of palladium with related metals, and their activity was supported with mechanistic studies. Moreover, the complete exclusion of Pd is also in focus. Very recently it was demonstrated that special amine organocatalysts could catalyse Suzuki-Miyaura coupling reaction. Here we show that in this recent transformation homeopathic palladium impurities and trace phosphorous species originated from the conditions used for the organocatalyst synthesis are responsible for the catalytic effect instead of the amine species. This finding confirms the power of palladium in cross-coupling and draw the attention of impurity effect in this field of chemical research. In this article, we represent general guidelines for elucidating the real catalyst of reactions.
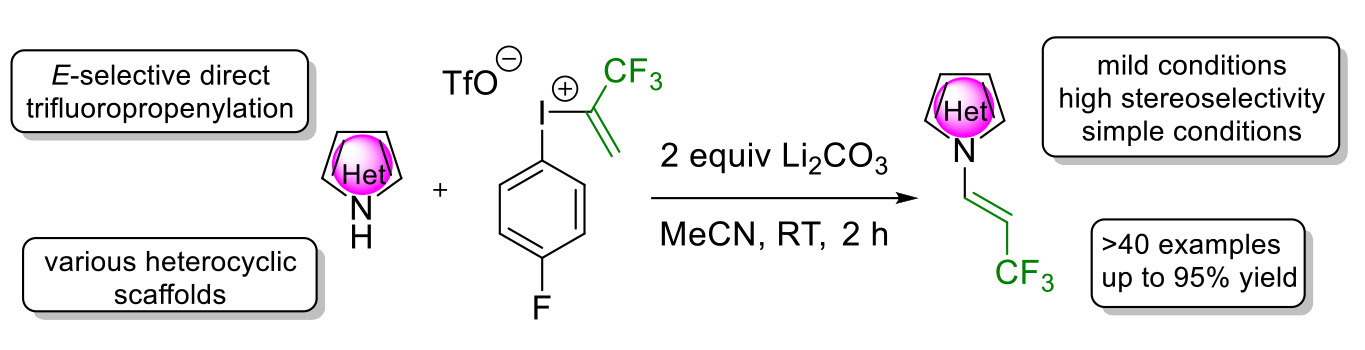
84. Stereoselective Direct N-Trifluoropropenylation of Heterocycles with a Hypervalent Iodonium Reagent
Stereoselective Direct N-Trifluoropropenylation of Heterocycles with a Hypervalent Iodonium Reagent, János T. Csenki, Ádám Mészáros, Zsombor Gonda, Zoltán Novák, Chem. Eur. J. 2021, 27, 15638-15643. DOI: 10.1002/chem.202102840 | [Full Text Link] [Supp. Info. Link]
The availability and synthesis of fluorinated enamine derivatives such as N-(3,3,3-trifluoropropenyl)heterocycles are challenging, especially through direct functionalization of the heterocyclic scaffold. Herein, we describe a stereoselective N-trifluoropropenylation method based on the use of a bench-stable trifluoropropenyl iodonium salt. This reagent enables the straightforward trifluoropropenylation of various N-heterocycles under mild reaction conditions, providing trifluoromethyl enamine type moieties with high stereoselectivity and efficiency.
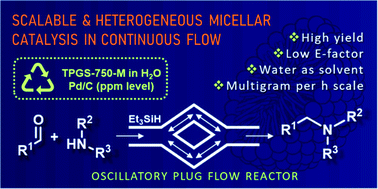
83. Continuous flow heterogeneous catalytic reductive aminations under aqueous micellar conditions enabled by an oscillatory plug flow reactor
Continuous flow heterogeneous catalytic reductive aminations under aqueous micellar conditions enabled by an oscillatory plug flow reactor, Michaela Wernik, Gellért Sipos, Balázs Buchholcz, Ferenc Darvas, Zoltán Novák, Sándor B. Ötvös, C. Oliver Kappe, Green Chem. 2021, 23, 5625-5632. DOI: 10.1039/D1GC02039K | [Full Text Link] [Supp. Info. Link]
Despite the fact that continuous flow processing exhibits well-established technical advances, aqueous micellar chemistry, a field that has proven extremely useful in shifting organic synthesis to sustainable water-based media, has mostly been explored under conventional batch-based conditions. This is particularly because of the fact that the reliable handling of slurries and suspensions in flow has been considered as a significant technical challenge. Herein, we demonstrate that the strategic application of an oscillatory plug flow reactor enables heterogeneous catalytic reductive aminations in aqueous micellar media enhancing mass transport and facilitating process simplicity, stability and scalability. The micellar flow process enabled a broad range of substrates, including amino acid derivatives, to be successfully transformed under reasonably mild conditions utilizing only very low amounts of Pd/C as a readily available heterogeneous catalyst. The preparative capabilities of the process along with the recyclability of the heterogenous catalyst and the aqueous reaction media were also demonstrated.
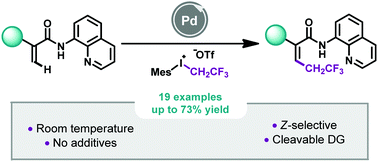
82. Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature
Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature, Louise Ruyet, Maria I. Lapuh, Vijay S. Koshti, Tamás Földesi, Philippe Jubault, Thomas Poisson, Zoltán Novák, Tatiana Besset, Chem. Commun. 2021, 57, 6241-6244. DOI: 10.1039/D1CC02007B | [Supp. Info. Link]
A straightforward 2,2,2-trifluoroethylation of acrylamides by Pd-catalyzed C–H bond activation was reported by using a fluorinated hypervalent iodine reagent as a coupling partner. At room temperature, this additive-free approach allowed the synthesis of Z-2,2,2-trifluoroethylated acrylamides (19 examples, up to 73% yield) in a stereoselective manner. Under these mild reaction conditions, the methodology turned out to be functional group tolerant and mechanistic studies gave us a better understanding of the transformation.
81. Application of industrially relevant HFO gases in organic syntheses
Application of industrially relevant HFO gases in organic syntheses, Bálint Varga, János Csenki, Balázs L. Tóth, Ferenc Béke, Zoltán Novák, András Kotschy, Synthesis 2021, 53, 4313-4326. DOI: 10.1055/a-1538-8344 | [Full Text Link]
Hydrofluoroolefin (HFO) gases are state-of-the-art cooling agents with widespread household and industrial applications. Considering their structural benefits these fluorous feedstocks gained attention of organic chemists in the last couple of years. In this short review we summarized the existing synthetic transformations of these gaseous starting material and present their applicability in the synthesis of fluorine containing organic molecules, which have potential importance as building blocks and reagents for diverse syntheses.
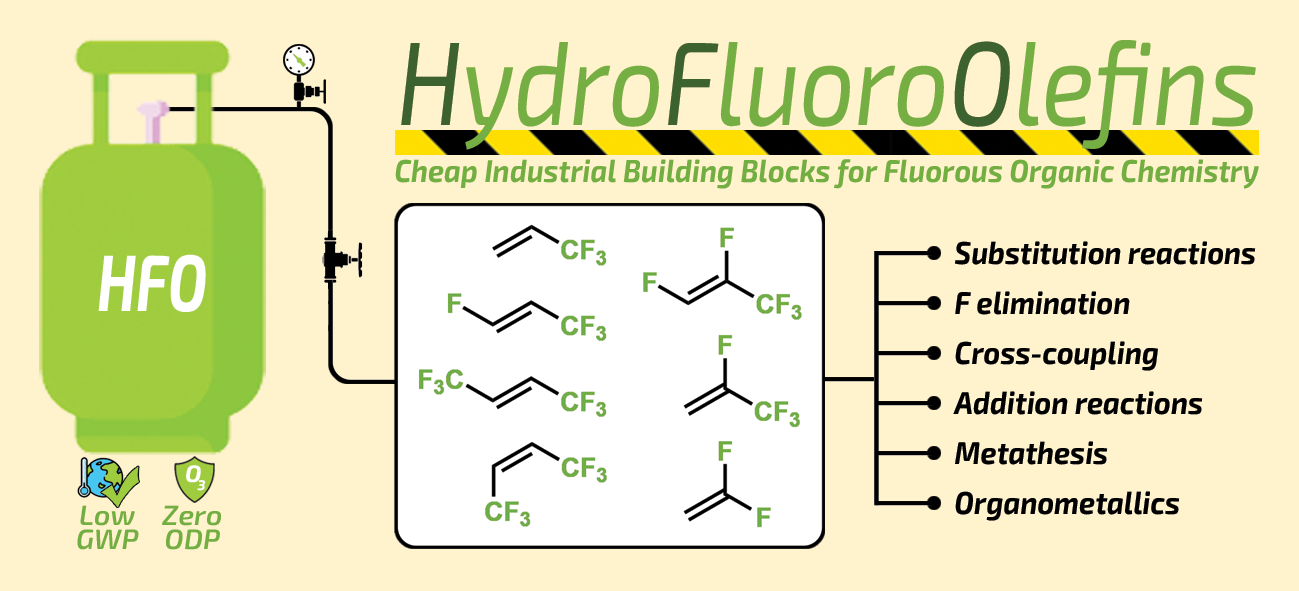
80. Synthesis and Photochemical Application of Hydrofluoroolefin (HFO) Based Fluoroalkyl Building Block
Synthesis and Photochemical Application of Hydrofluoroolefin (HFO) Based Fluoroalkyl Building Block, Bálint Varga, Balázs L. Tóth, Ferenc Béke, János T. Csenki, András Kotschy, Zoltán Novák, Org. Lett. 2021, 23, 4925–4929. DOI: 10.1021/acs.orglett.1c01709 | [Full Text Link] [Supp. Info. Link]
A novel fluoroalkyl iodide was synthesized on multigram scale from refrigerant gas HFO-1234yf as cheap industrial starting material in a simple, solvent-free, and easily scalable process. We demonstrated its applicability in a metal-free photocatalytic ATRA reaction to synthesize valuable fluoroalkylated vinyl iodides and proved the straightforward transformability of the products in cross-coupling chemistry to obtain conjugated systems.
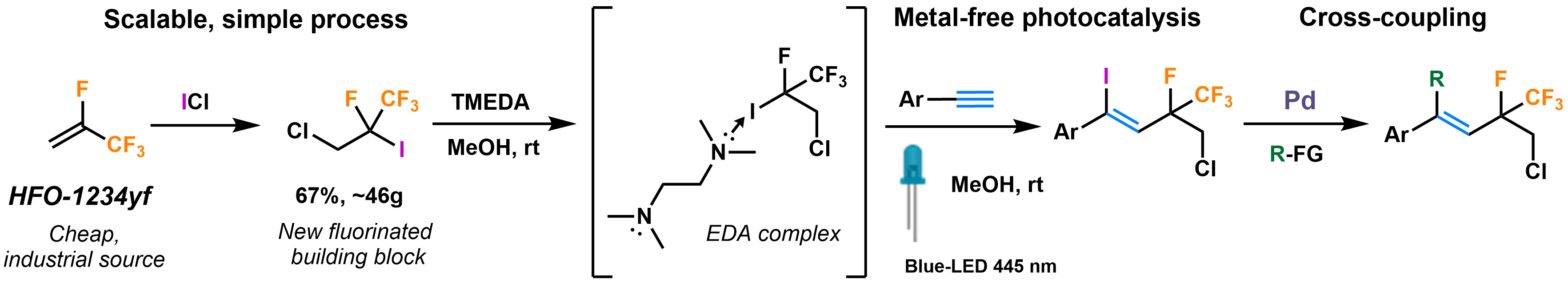
79. The Ortho Effect in Directed C-H Activation
The Ortho Effect in Directed C–H Activation, Balázs L. Tóth, Anna Monory, Orsolya Egyed, Attila Domján, Attila Bényei, Bálint Szathury, Zoltán Novák, András Stirling, Chem. Sci. 2021, 12, 5152-5163. Open Access. DOI: 10.1039/D1SC00642H | [Full Text Link] [Supp. Info. Link] [Full Interactive Database (XLSX)] [Crystal Data (CIF)] [Structures 1 (XYZ)] [Structures 2 (XYZ)]
The success of transition metal-catalysed ortho-directed C–H activation is often plagued by the effects of undesirable interactions between the directing group (DG) and other groups introduced into the aromatic core of the substrate. In particular, when these groups are in neighbouring positions, their interactions can affect profoundly the efficacy of the C–H activation by transition metals. In this work we introduce a simple substrate-only-based model to interpret the influence of steric hindrance of a group in ortho position to the DG in directed ortho-C–H bond activation reactions, and coined the term Ortho Effect (OE) for such situations. We consider simple descriptors such as torsion angle and torsional energy to predict and explain the reactivity of a given substrate in directed C–H activation reactions. More than 250 examples have been invoked for the model, and the nature of the ortho effect was demonstrated on a wide variety of structures. In order to guide organic chemists, we set structural and energetic criteria to evaluate a priori the efficiency of the metalation step which is usually the rate-determining event in C–H activations, i.e. we provide a simple and general protocol to estimate the reactivity of a potential substrate in C–H activation. For borderline cases these criteria help set the minimum reaction temperature to obtain reasonable reaction rates. As an example for the practical applicability of the model, we performed synthetic validations via palladium-catalysed 2,2,2-trifluoroethylation reactions in our lab. Furthermore, we give predictions for the necessary reaction conditions for several selected DGs.
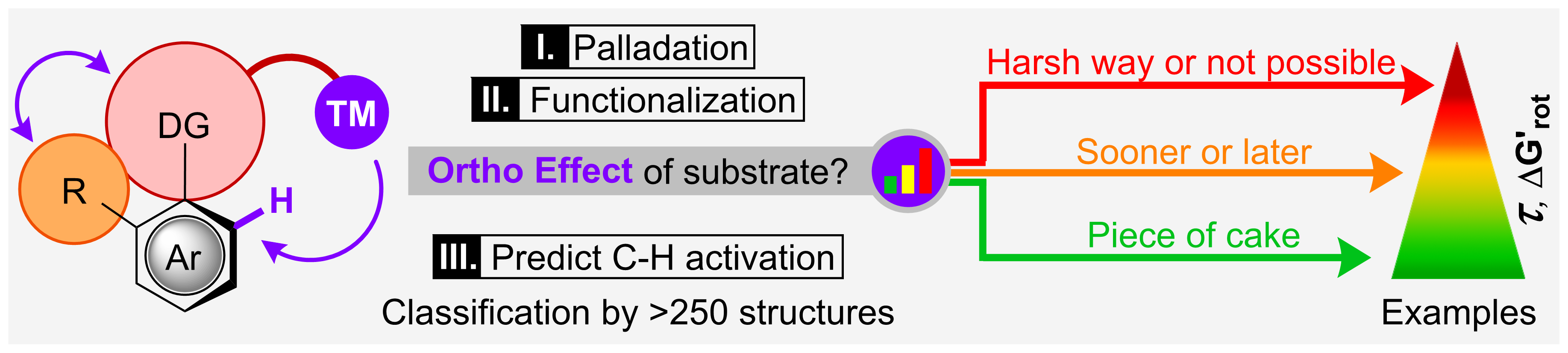
78. Vicinal difunctionalization of carbon-carbon double bond for the platform synthesis of trifluoroalkyl amines
Vicinal difunctionalization of carbon–carbon double bond for the platform synthesis of trifluoroalkyl amines, Ferenc Béke, Ádám Mészáros, Ágnes Tóth, Bence Béla Botlik, Zoltán Novák, Nat. Commun. 2020, 5924. DOI: 10.1038/s41467-020-19748-z | [Full Text Link] [Supp. Info. Link]
Regioselective vicinal diamination of carbon–carbon double bonds with two different amines is a synthetic challenge under transition metal-free conditions, especially for the synthesis of trifluoromethylated amines. However, the synthesis of ethylene diamines and fluorinated amine compounds is demanded, especially in the pharmaceutical sector. Herein, we demonstrate that the controllable double nucleophilic functionalization of an activated alkene synthon originated from a trifluoropropenyliodonium salt with two distinct nucleophiles enables the selective synthesis of trifluoromethylated ethylene amines and diamines on broad scale with high efficiency under mild reaction conditions. Considering the chemical nature of the reactants, our synthetic concept methodology brings forth an efficient methodology and provides versatile access to highly fluorinated amines.
77. Photocatalytic Palladium-Catalyzed Fluoroalkylation of Styrene Derivatives
Photocatalytic Palladium-Catalyzed Fluoroalkylation of Styrene Derivatives, Réka Adamik, Tamás Földesi, Zoltán Novák, Org. Lett. 2020, 22, 8091-8095. DOI: 10.1021/acs.orglett.0c03043 | [Full Text Link] [Supp. Info. Link]
A visible light induced palladium-catalyzed fluoroalkylation method was developed. The Heck-type alkyl coupling reaction enables the introduction of trifluoroethyl, difluoroethyl and other fluoroalkyl fragment into styrenes under mild reaction conditions without the use of additional photosensitizers and ensures access to fluoroalkylated olefins on a broad scale.
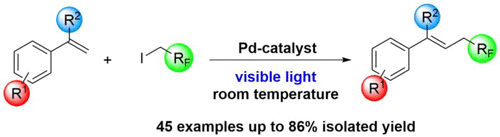
76. Synthesis, Structural Analysis and Application of Aryl‐Diadamantyl Phosphine ligands in Palladium Catalyzed Cross‐Coupling Reactions
Synthesis, Structural Analysis and Application of Aryl‐Diadamantyl Phosphine ligands in Palladium Catalyzed Cross‐Coupling Reactions, Ádám Sinai, Dániel Csaba Simkó, Fruzsina Szabó, Attila Paczal, Tamás Gáti, Attila Bényei, Zoltán Novák, András Kotschy, Eur. J. Org. Chem. 2020, 9, 1122-1128. DOI: 10.1002/ejoc.201901834 | [Full Text Link] [Supp. Info. Link]
Synthesis, temperature dependent NMR structure investigation and utilization of a new, stable and easily accessible aryl‐diadamantylphosphine ligand family is reported. The bulky and electron rich phosphorous center of the ligand enhances the catalytic activity of palladium in cross‐coupling reactions of sterically demanded ortho substituted aryl halides. In our study we demonstrated the synthetic applicability of the new phosphine ligands in Buchwald‐Hartwig and tosyl hydrazone coupling reactions.
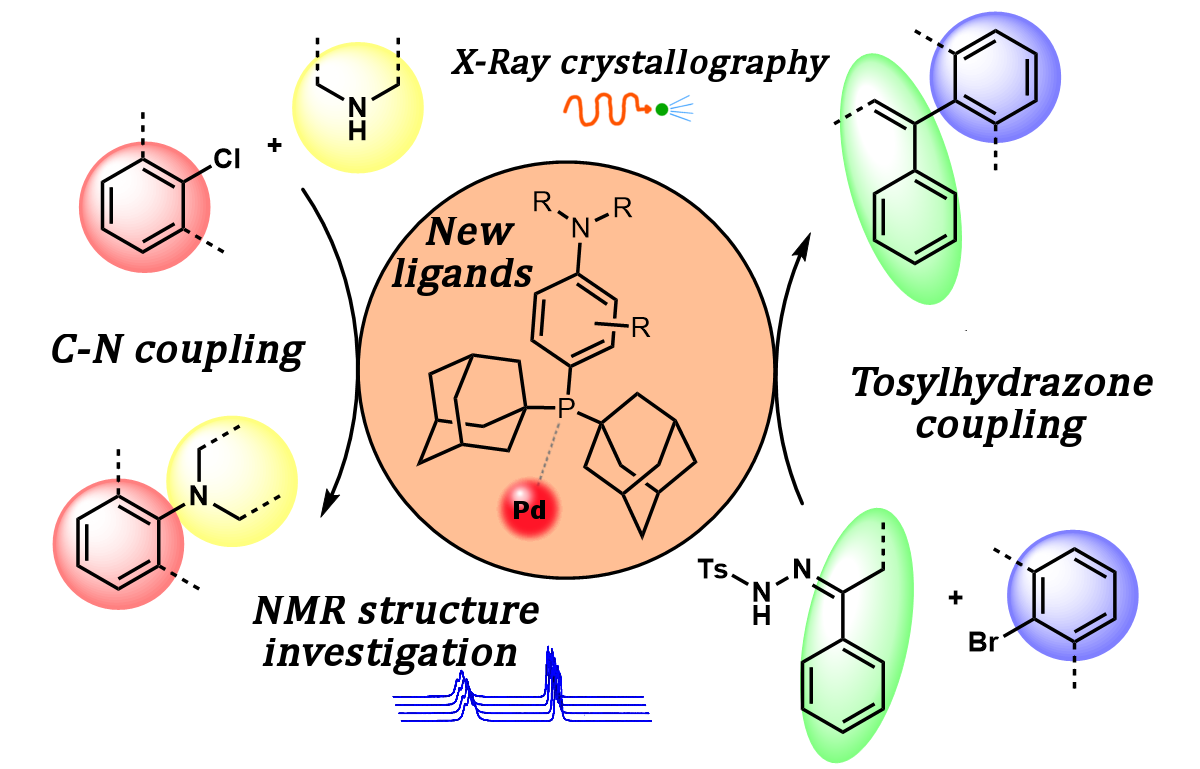
75. Microstructural Investigation of Nanocrystalline Hydrogen-Storing Mg-Titanate Nanotube Composites Processed by High-Pressure Torsion
Microstructural Investigation of Nanocrystalline Hydrogen-Storing Mg-Titanate Nanotube Composites Processed by High-Pressure Torsion, Marcell Gajdics, Tony Spassov, Viktória Kovács Kis, Ferenc Béke, Zoltán Novák, Erhard Schafler, Ádám Révész, Energies 2020, 13, 563. DOI: 10.3390/en13030563 | [Full Text Link]
A high-energy ball milling and subsequent high-pressure torsion method was applied to synthesize nanocrystalline magnesium samples catalyzed by TiO2 or titanate nanotubes. The microstructure of the as-milled powders and the torqued bulk disks was characterized by X-ray diffraction. The recorded diffractograms have been evaluated by the convolutional multiple whole profile fitting algorithm, which provided microstructural parameters (average crystal size, crystallite size distribution, average dislocation density). The morphology of the nanotube-containing disks has been examined by high-resolution transmission electron microscopy. The effect of the different additives and preparation conditions on the hydrogen absorption behavior was investigated in a Sieverts’-type apparatus. It was found that the ball-milling route has a prominent effect on the dispersion and morphology of the titanate nanotubes, and the absorption capability of the Mg-based composite is highly dependent on these features.
Keywords: ball milling; high-pressure torsion; magnesium; titanate nanotubes; hydrogen storage
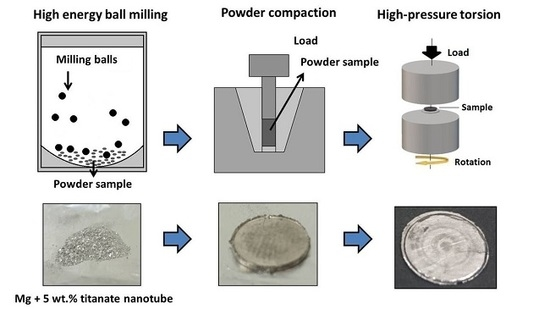
74. Development of Ni‐Ir dual photocatalytic Liebeskind coupling of sulfonium salts for the synthesis of 2‐benzylpyrrolidines
Development of Ni‐Ir dual photocatalytic Liebeskind coupling of sulfonium salts for the synthesis of 2‐benzylpyrrolidines, Bálint Varga, Zsombor Gonda, Balázs L. Tóth, András Kotschy, Zoltán Novák, Eur. J. Org. Chem. 2020. 10, 1466-1471. DOI: 10.1002/ejoc.201900957 | [Full Text Link] [Supp. Info. Link]
A new method has been developed for the synthesis of 2‐benzylpyrrolidines utilizing cross‐coupling and photoredox catalysis. Using a well‐established dual Ni‐Ir system, we were able to successfully couple benzylsulfonium salts with a proline utilizing radical forming through CO2 extrusion. This enabled the simple one step synthesis of 2‐benzylpyrrolidines from stable inexpensive starting materials.
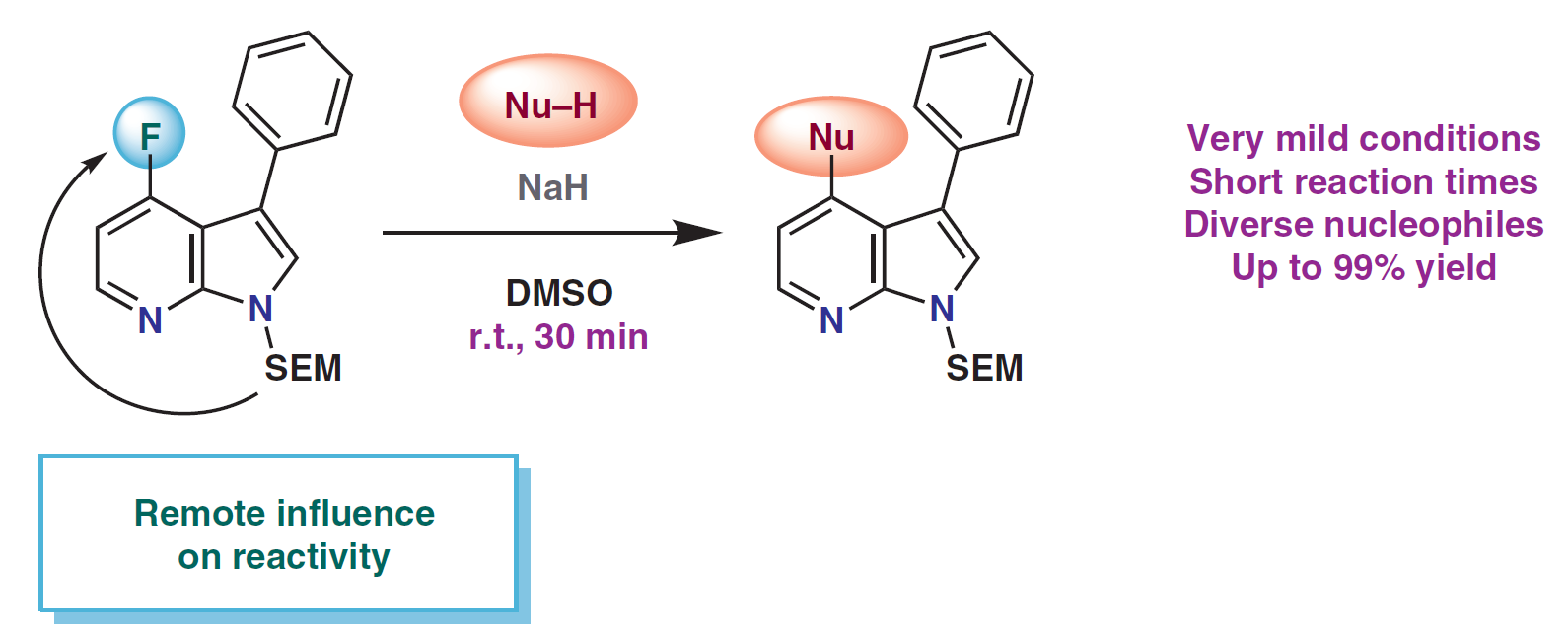
73. Efficient Synthesis of 3,4-Disubstituted 7-Azaindoles Employing SEM as a Dual Protecting–Activating Group
Efficient Synthesis of 3,4-Disubstituted 7-Azaindoles Employing SEM as a Dual Protecting–Activating Group, Piroska Gyárfás, János Gerencsér, Warren S. Wade, László Ürögdi, Zoltán Novák, S. Todd Meyer, Synlett 2019, 30, 2273-2278. DOI: 10.1055/s-0039-1690735 | [Full Text Link] [Supp. Info. Link]
An efficient method for nucleophilic aromatic substitution on 7-azaindoles has been developed. The reaction is facilitated by the unique dual influence of SEM as both protecting and activating group, permitting mild conditions and short reaction times that are compatible with sensitive functional groups. The method is suitable for the synthesis of a broad range of products, most notably ethers.
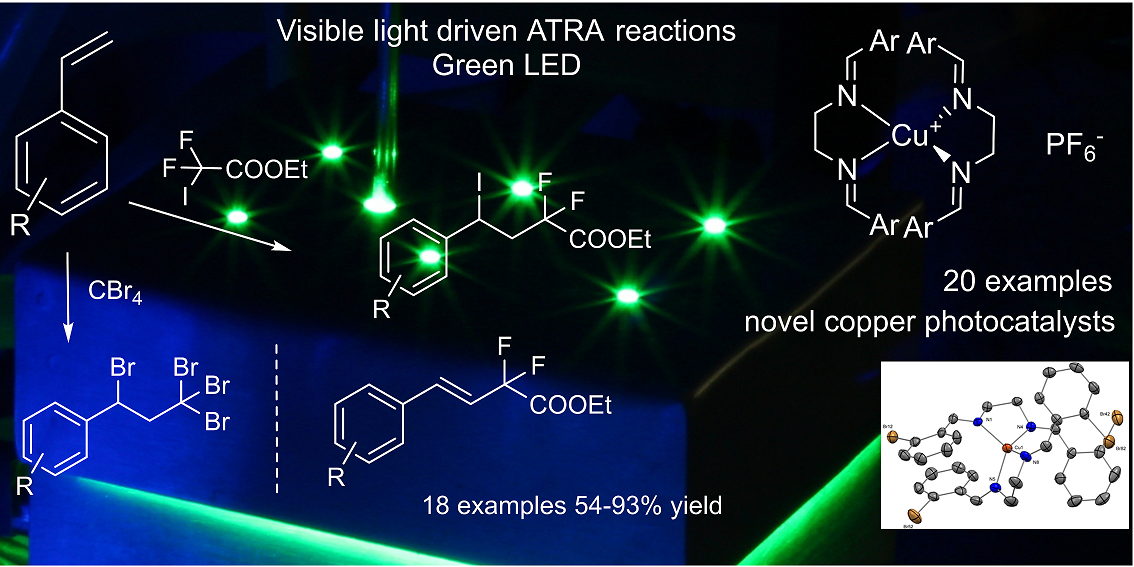
72. Design and application of diimine-based copper(I) complexes in photoredox catalysis
Design and application of diimine-based copper(I) complexes in photoredox catalysis, Tamás Földesi, Réka Adamik, Gellert Sipos, Bálint Nagy, Balázs L. Tóth, Attila Benyei, Krisztina Szekeres, Győző Láng, Attila Demeter, Timothy Peelen, Zoltán Novák, Org. Biomol. Chem. 2019, 17, 8343-8347. DOI: 10.1039/C9OB01331H | [Full Text Link] [Supp. Info. Link, CIF]
Structurally different bis(imino)copper(I) complexes were prepared in a highly modular manner and utilized as copper-based photocatalysts in ATRA reactions of styrenes and alkyl halides. The new photocatalysts showed good catalytic activity and ensured efficient chemical transformations.
Front cover of the issue: Org. Biomol. Chem.2019,17, 8263-8264. DOI:10.1039/C9OB90149C | [Full Text Link]

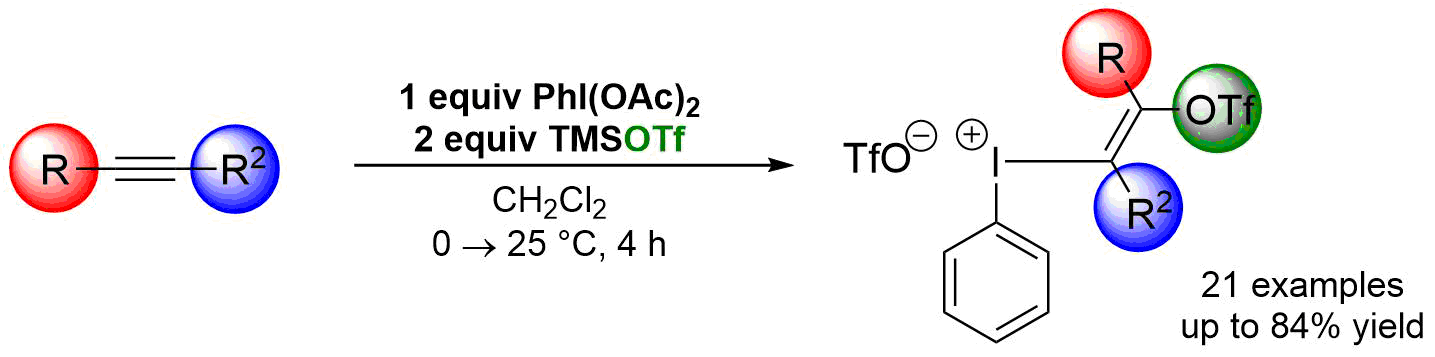
71. Synthesis of Multifunctional Aryl(trifloxyalkenyl)iodonium Triflate Salts
Synthesis of Multifunctional Aryl(trifloxyalkenyl)iodonium Triflate Salts, Balázs L. Tóth, Ferenc Béke, Orsolya Egyed, Attila Bényei, András Stirling, Zoltán Novák, ACS Omega 2019, 45, 9188-9197. DOI: 10.1021/acsomega.9b00728 | [Full Text Link] [Supp. Info. Link, CIF]
A convenient procedure for the synthesis of aryl(trifloxyalkenyl)iodonium triflate salts from commercially available (diacetoxyiodo)benzene, trimethylsilyl trifluoromethanesulfonate, and acetylenes under mild conditions was developed. The obtained multifunctional hypervalent vinyliodonium salts equipped with electrophilic and nucleophilic functions could serve as novel C2 synthons for organic transformations. The structure of the iodonium salts was identified by multidimensional NMR spectroscopy and X-ray crystallography.
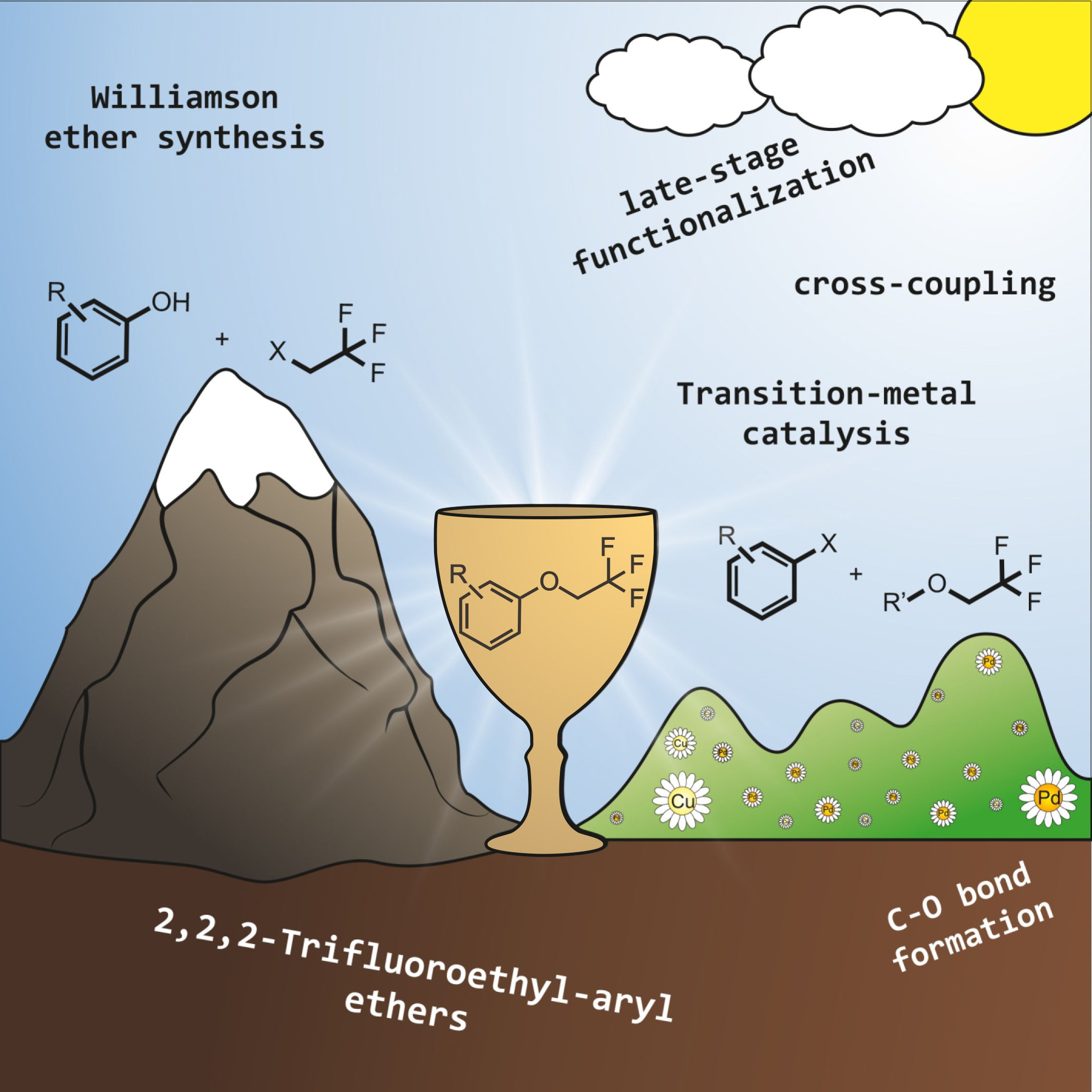
70. Synthesis of aryl‐ and heteroaryl‐trifluoroethyl ethers: aims, challenges and new methodologies
Synthesis of aryl‐ and heteroaryl‐trifluoroethyl ethers: aims, challenges and new methodologies, Bálint Pethő, Zoltán Novák, Asian J. Org. Chem. 2019, 568-575. DOI: 10.1002/ajoc.201800414 | [Full Text Link]
Focus Review: Fluorinated substances have outstanding importance in most fields of the chemical industry, from agrochemicals and material science to pharmaceutical research. Although aryl‐ and heteroaryl‐trifluoroethyl ethers have widespread applications, predominantly as drug molecules, their preparation was limited to classical synthetic methodologies until the last decade. The transition metal catalyzed cross coupling reactions offered novel synthetic approaches for the facile access of trifluoroethoxylated aromatic compounds. The aim of this review is to give a good overview on recent advances in the synthesis of aryl‐trifluoroethyl ethers from a practical point of view for synthetic organic chemists working in the field of pharmaceutical industry and academia.
69. Transition Metal‐Catalyzed Reactions with Iodine(III) Reagents
Transition Metal‐Catalyzed Reactions with Iodine(III) Reagents, Gergely L. Tolnai, Zsombor Gonda, Zoltán Novák, PATAI'S Chemistry of Functional Groups, Hypervalent Halogen Compounds (2018) | DOI: 10.1002/9780470682531.pat0961 | [Full Text Link]
Transition metal‐catalyzed functionalizations and heterocycle formations with hypervalent iodine reagents are discussed. The most important arylations of aromatic and heteroaromatic systems via cross‐coupling with diaryliodonium salts and C-H bond activation are collected and described, focusing on the reaction mechanisms and the most important applications. Although the C-C bond formations are the major parts of these transformations, the carbon–heteroatom bond‐forming reactions are also presented from two aspects. While the iodonium species provides the aryl group and reacts with nucleophilic heteroatom (oxygen or nitrogen), in the other approach, (diacetoxyiodo)benzene (DIB) serves as heteroatom donor to achieve acetoxylations of aromatic rings or the formation of three‐membered heterocycles (epoxides and aziridines) from unsaturated species. Due to the interaction of iodonium salts and special functional groups of the substrate, the initial C-C or C-heteroatom bond formation could result in a reactive intermediate, which could induce a multiple bond‐formation relay to access special heterocyclic molecules.
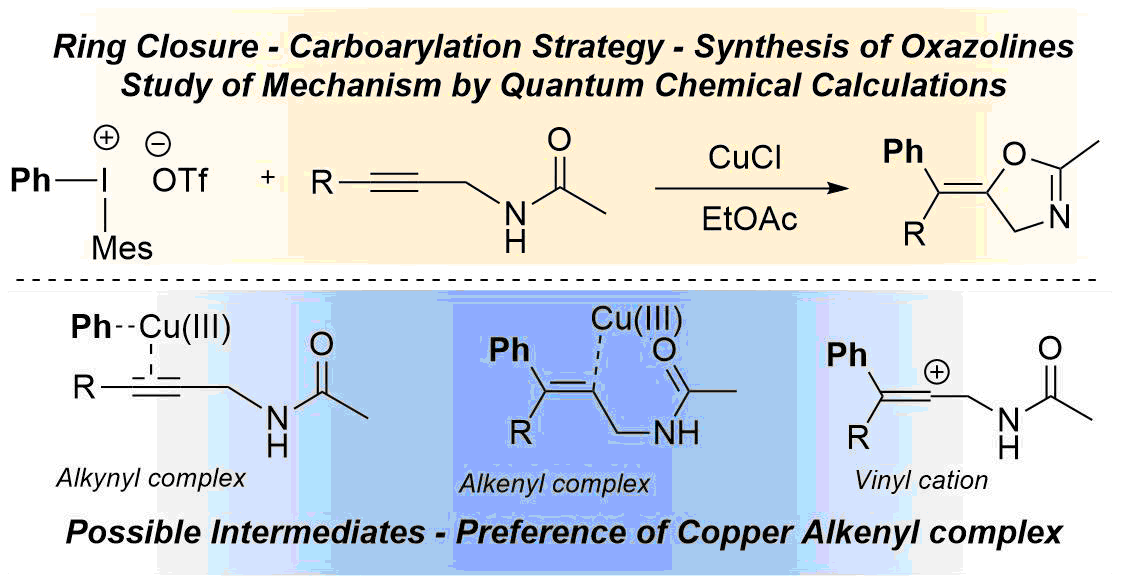
68. DFT Calculations on the Mechanism of Copper-Catalysed Tandem Arylation–Cyclisation Reactions of Alkynes and Diaryliodonium Salts
DFT calculations on the mechanism of copper-catalysed tandem arylation–cyclisation reactions of alkynes and diaryliodonium salts, Tamás Károly Stenczel, Ádám Sinai, Zoltán Novák, András Stirling. Beilstein. J. Org. Chem. 2018,14, 1743-1749. DOI: 10.3762/bjoc.14.148 | [Full Text Link] [Supp. Info. Link]
We present a computational mechanistic study on the copper(III)-catalysed carboarylation–ring closure reactions leading to the formation of functionalised heterocycles. We have performed DFT calculations along selected routes and compared their free energy profiles. The calculations considered two viable options for the underlying mechanism which differ in the order of the oxazoline ring formation and the aryl transfer steps. In our model transformation, it was found that the reaction generally features the aryl transfer–ring closing sequence and this sequence shows very limited sensitivity to the variation of the substituent of the reactants. On the basis of the mechanism the origin of the stereoselectivity is ascribed to the interaction of the Cu ion with the oxazoline oxygen driving the ring-closure step selectively.
Keywords: catalysis; DFT calculation; iodonium salts; reaction mechanism; tandem arylation–cyclisation
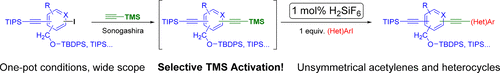
67. Catalytic Activation of Trimethylsilylacetylenes: A One-Pot Route to Unsymmetrical Acetylenes and Heterocycles
Catalytic Activation of Trimethylsilylacetylenes: A One-Pot Route to Unsymmetrical Acetylenes and Heterocycles, Dániel Lasányi, Ádám Mészáros, Zoltán Novák, Gergely L. Tolnai, J. Org. Chem. 2018. 83, 8281-8291. DOI: 10.1021/acs.joc.8b00998 | [Full Text Link] [Supp. Info. Link]
For the synthesis of unsymmetrical acetylenes, a Sonogashira coupling–deprotection–Sonogashira coupling reaction sequence is often used. Removal of protecting groups requires harsh conditions or an excess of difficult to handle and expensive reagents. Herein, we disclose a novel catalytic method for the selective deprotection of trimethylsilylacetylenes in Sonogashira reaction. The reagent hexafluorosilicic acid, an inexpensive nontoxic compound, was used to promote the selective desilylation. This method enables the efficient synthesis of unsymmetric acetylenes with other silylated functional groups present. Further possibilities of the method were explored by synthesis of heterocycles.
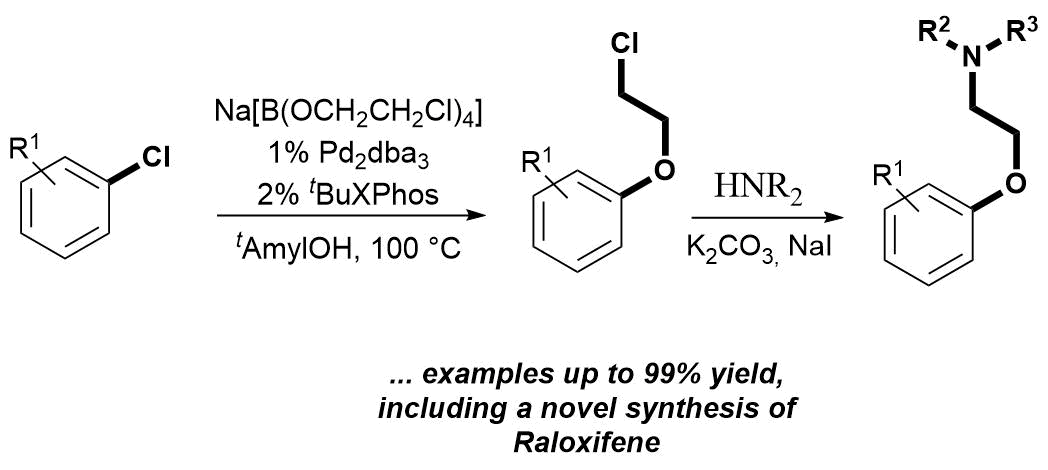
66. Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker
Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker, Bálint Pethő, Dóra Vangel, János Tivadar Csenki, Márton Zwillinger, Zoltán Novák, Org. Biomol. Chem. 2018. 16, 4895-4899. DOI: 10.1039/C8OB01146 | [Full Text Link] [Supp. Info. Link]
A novel disconnection based on cross-coupling chemistry was designed to access pharmaceutically relevant aryl-aminoethyl ethers. The developed palladium-catalyzed functionalization of aryl- and heteroaryl chlorides with sodium tetrakis-(2-chloroethoxy)-borate salt is orthogonal to the simple nucleophilic replacement of the chloro function of the ethylene linker. The transformation enables efficient 2-chloroethoxylation in the absence of additional external base. Subsequent amine substitution of the alkyl halide affords 2-aminoethoxy arenes. The applicability of this method was demonstrated through the synthesis of various aryl- and heteroaryl-alkyl ethers, including intermediates of marketed drug molecules.
65. Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring
Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring, Ádám Mészáros, Anna Székely, András Stirling, Zoltán Novák, Angew. Chem. Int. Ed. 2018, 57, 6643-6647. DOI: 10.1002/anie.201802347 | [Full Text Link] [Supp. Info. Link]
Synthesis of fluorinated compounds and their use as pharmaceutical ingredients or synthetic building blocks are in the focus of chemical and medicinal research. However, the efficient synthesis of trifluoromethylated nitrogen heterocycles sometimes are challenging. Herein, we disclose a simple aziridination process which relies on the use of amines and novel alkenyl synthon for the access of trifluoromethylated strained heterocycle. With the utilization of a newly designed, bench stable but highly reactive hypervalent alkenyl iodonium species, the three membered heterocyclic ring can be constructed from simple amines without structural limitation with high efficiency under mild conditions in the absence of transition metal catalysts. The special reactivity of the new trifluoropropenyl synthon toward nucleophilic centres could be exploited in more general cyclization and alkenylation reactions in the future.
64. Sulfonium Salts as Alkylating Agents for Palladium-Catalyzed Direct Ortho Alkylation of Anilides and Aromatic Ureas
Sulfonium Salts as Alkylating Agents for Palladium-Catalyzed Direct Ortho Alkylation of Anilides and Aromatic Ureas, Dániel Cs. Simkó, Péter Elekes, Vivien Pázmándi, Zoltán Novák, Org. Lett. 2018, 20, 676-679. DOI: 10.1021/acs.orglett.7b03813 | [Full Text Link] [Supp. Info. Link]
A novel method for the ortho alkylation of acetanilide and aromatic urea derivatives via C–H activation was developed. Alkyl dibenzothiophenium salts are considered to be new reagents for the palladium-catalyzed C–H activation reaction, which enables the transfer of methyl and other alkyl groups from the sulfonium salt to the aniline derivatives under mild catalytic conditions.
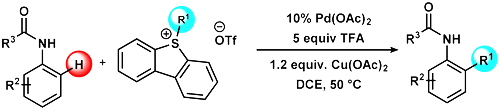
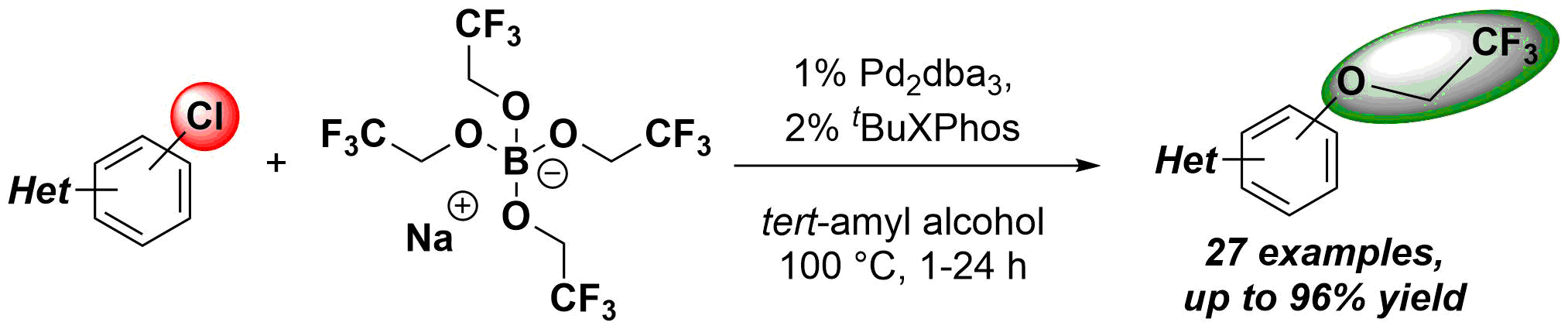
63. Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil
Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil, Bálint Pethő, Márton Zwillinger, János Csenki, Anna Káncz, Balázs Krámos, Judit Müller, György Tibor Balogh, Zoltán Novák, Chem. Eur. J. 2017, 62, 15628-15632. DOI: 10.1002/chem.201704205 | [Full Text Link] [Supp. Info. Link]
A simple and convenient method was developed for the introduction of 2,2,2-trifluoroethoxy group to various aromatic and heteroaromatic systems. The novel process utilizes aromatic chlorides as substrates, and tetrakis(2,2,2-trifluoroethoxy) borate salt as an inexpensive and readily available fluoroalkoxy source in a palladium-catalyzed cross-coupling reaction. The power of the developed methodology was demonstrated in the synthesis of fluorous derivative of Sildenafil.
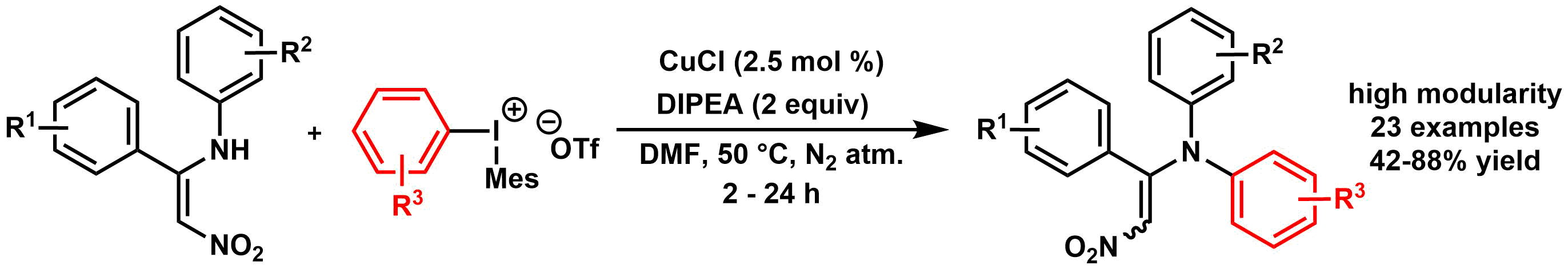
62. Copper-Catalyzed N-Arylation of Nitroenamines with Diaryliodonium Salts
Copper-Catalyzed N-Arylation of Nitroenamines with Diaryliodonium Salts, Klára Aradi, Ádám Mészáros, Balázs L. Tóth, Zoltán Vincze, Zoltán Novák, J. Org. Chem. 2017, 82, 11752-11764. DOI: 10.1021/acs.joc.7b01591 | [Full Text Link] [Supp. Info. Link]
A novel synthetic methodology was developed for the N-arylation of nitroenamine derivatives utilizing diaryliodonium triflates and copper (I) chloride as a catalyst. The procedure enables the ease aryl transfer from the hypervalent species under mild catalytic conditions with unusual heteroatom preference and high efficiency.
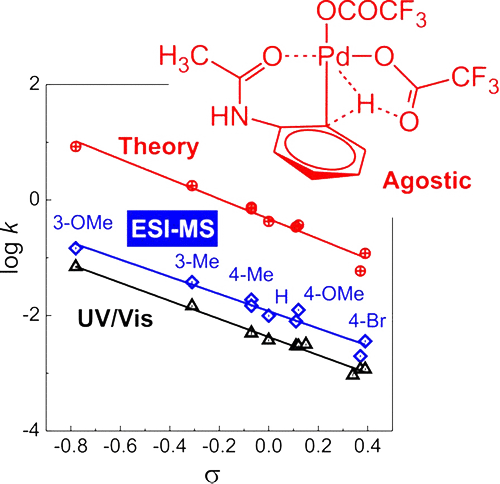
61. Palladium-Catalyzed C–H Activation: Mass Spectrometric Approach to Reaction Kinetics in Solution
Palladium-Catalyzed C–H Activation: Mass Spectrometric Approach to Reaction Kinetics in Solution, Jiří Váňa, Thibault Terencio, Vladimir Petrović, Orsolya Tischler, Zoltán Novák, Jana Roithová, Organometallics 2017, 36, 2072-2080. DOI: 10.1021/acs.organomet.6b00960 | [Full Text Link] [Supp. Info. Link, XYZ]
We report a new method for determination of rate constants of processes in solution using electrospray ionization mass spectrometry (ESI-MS). The investigated reaction is C–H activation of acetanilides by palladium(II)trifluoroacetate leading to stable organopalladium complexes. The rate constants can be determined from an experiment with a couple of differently substituted acetanilides being in competition for being activated by the palladium salt. The formed organopalladium complexes can be detected by ESI-MS. The time dependence is achieved by adding one of the acetanilides to the reaction mixture with a time delay. The kinetics can be then evaluated from the evolution of the ratio of the ESI-MS signals of differently substituted complexes as a function of the time delay. The Hammett analysis of the rate constants obtained for a series of meta- and para-substituted acetanilides provides a ρ value of −1.5, which is in agreement with values reported for similar C–H activations. We have also investigated the very same reaction with UV–vis spectroscopy that gave us about three times smaller rate constants but the same trend with the ρ value of −1.6. The rate constants determined by ESI-MS are directly linked to the occurrence of organopalladium complexes, whereas the UV–vis data are associated with an absorption spectra change that could involve more reaction steps. DFT calculations support the interpretation of the reaction mechanism as cyclopalladation and provide the ρ value in the same range. The rate-determining step corresponds to the agostic C–H transition structure.
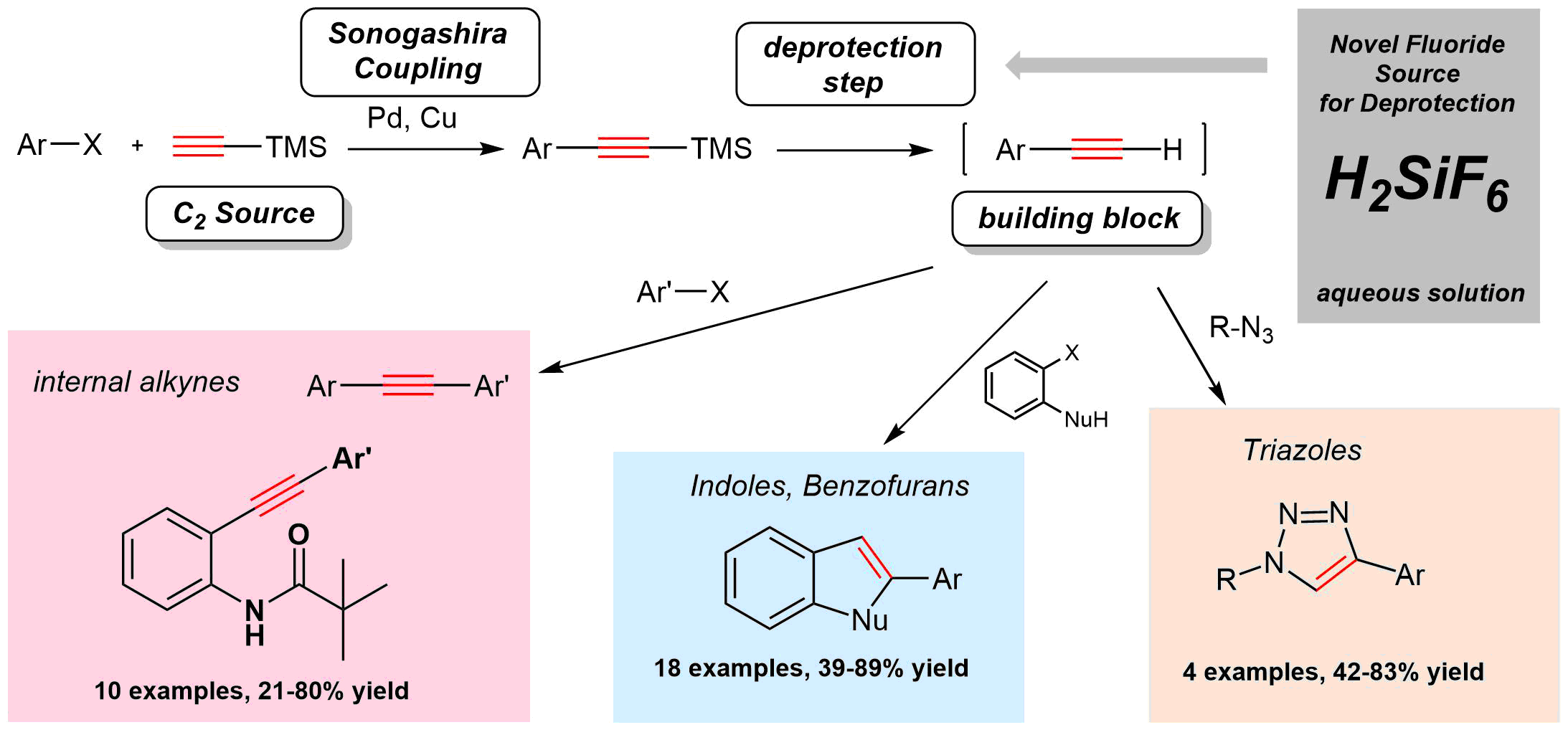
60. Hexafluorosilicic Acid as a Novel Reagent for the Desilylation of Silylacetylenes: Application in Sequential Sonogashira Coupling and Click Reaction
Hexafluorosilicic Acid as a Novel Reagent for the Desilylation of Silylacetylenes: Application in Sequential Sonogashira Coupling and Click Reaction, Ádám Sinai, Ádám Mészáros, Ádám Balogh, Márton Zwillinger, Zoltán Novák, Synthesis 2017, 49, 2374-2388. DOI: 10.1055/s-0036-1588981 | [Full Text Link] [Supp. Info. Link]
Key words: alkynes - azides - cycloaddition - click reaction - cross-coupling - fluoride - palladium - heterocycles
Hexafluorosilicic acid was utilized as a novel, cheap, readily available, and environmentally benign alternative reagent for the desilylation of 1-trimethylsilylacetylenes. The applicability of the aqueous solution of the hexafluorosilicic acid was demonstrated in the sequential coupling of aryl halides and ethynyltrimethylsilane to afford internal acetylenes, benzofurans, and triazoles in one-pot Sonogashira–Sonogashira and Sonogashira–CuAAC reactions.
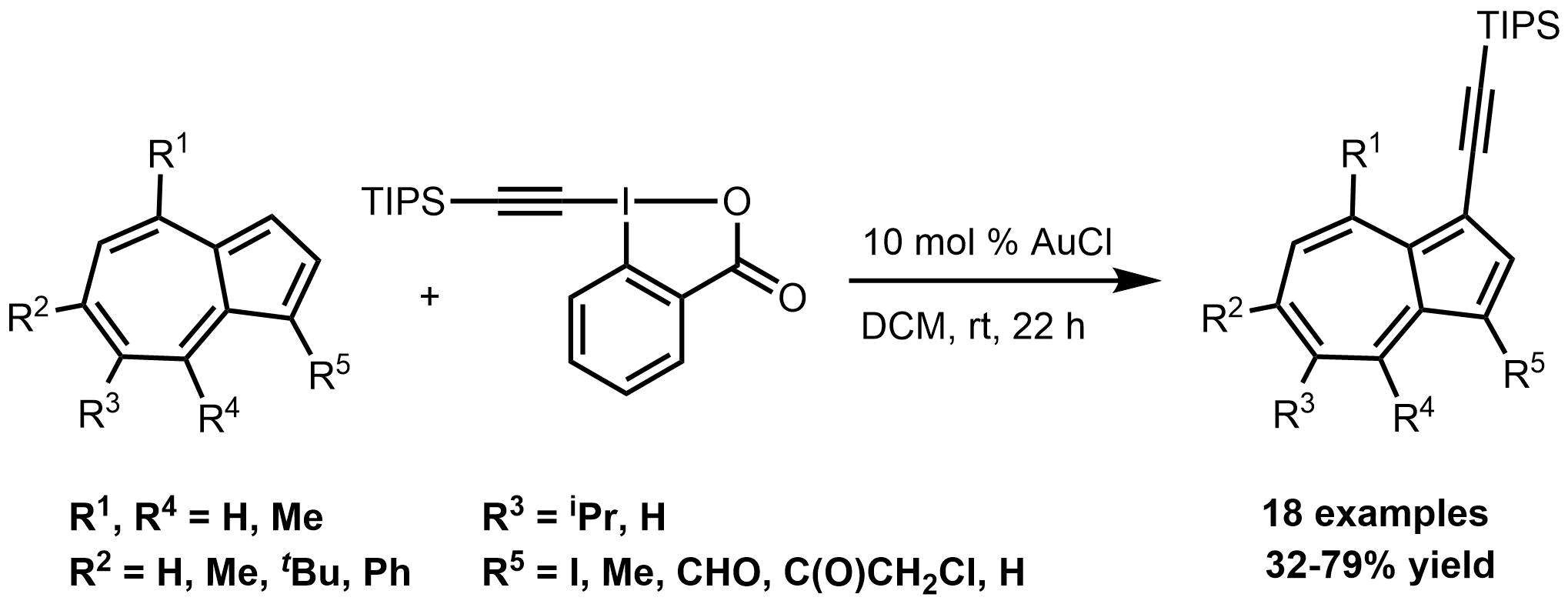
59. Gold-Catalyzed Direct Alkynylation of Azulenes
Gold-Catalyzed Direct Alkynylation of Azulenes, Anna Székely, Áron Péter, Klára Aradi, Gergely L. Tolnai, Zoltán Novák, Org. Lett. 2017, 19, 954-957. DOI: 10.1021/acs.orglett.7b00259 | [Full Text Link] [Supp. Info. Link]
A novel catalytic method for the direct C-H alkynylation of azulenes was developed. The gold catalyzed functionalization of this special carbacycle iwas achieved with hypervalent iodonium reagent TIPS-EBX under mild reaction conditions. With the aid of the developed procedure, several TIPS alkynylated azulene derivatives were synthesized bearing important func-tional groups for further functionalization.
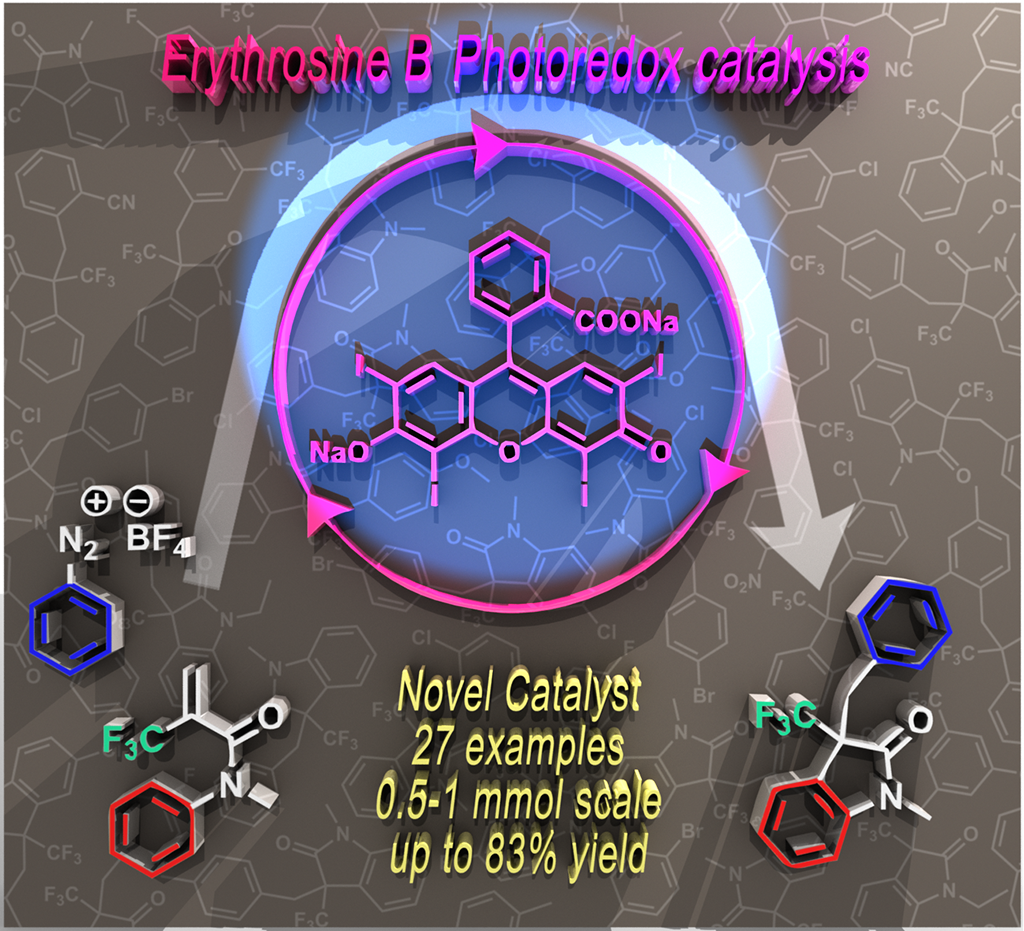
58. Erythrosine B catalyzed visible-light photoredox arylation-cyclization of N-alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(trifluoromethyl)indolin-2-one derivatives
Erythrosine B catalyzed visible-light photoredox arylation-cyclization of N-alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(trifluoromethyl)indolin-2-one derivatives, Zsombor Gonda, Ferenc Béke, Orsolya Tischler, Milán Petró, Zoltán Novák, Balázs L. Tóth, Eur. J. Org. Chem. 2017, 15, 2112-2117. DOI: 10.1002/ejoc.201601493 | [Full Text Link] [Supp. Info. Link]
Special Issue: Photoredox Catalysis
3-Trifluoromethyl-indoline-2-one derivatives were prepared in a visible-light photocatalytic transformation of acrylamides. The arylation-ring closure was initiated by light induced aryl radical generation from aryl diazonium salts with the utilization of erythrosine B as novel organic photocatalyst.
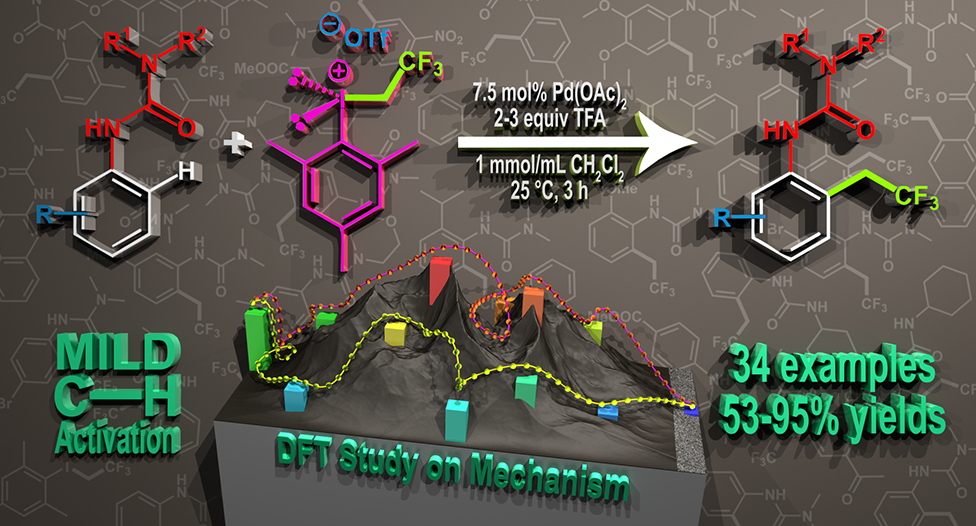
57. Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions
Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions, Szabolcs Kovács, Balázs L. Tóth, Gábor Borsik, Tamás Bihari, Nóra V. May, András Stirling, Zoltán Novák, Adv. Synth. Catal. 2017, 359, 527-532. DOI: 10.1002/adsc.201601136 | [Full Text Link] [Supp. Info. Link]
Abstract: Development of direct late-stage installation of alkyl groups into aromatic systems is an important and challenging task of current organic chemistry. In spite of the existing functionalization methods in organic chemistry for the substitution reactions on aromatic systems, the direct alkylation of aromatic ureas is unknown. Herein, as a first example we report a novel palladium catalyzed fluoroalkylation process by C−H activation for the access of ortho trifluoroethylated aromatic ureas. The application of novel, highly active trifluoroethyl(mesityl)iodonium salt enables the efficient introduction of the trifluoroethyl group at 25 °C in 3 hours in high yields (up to 95%) with good functional group tolerance. DFT calculations have revealed a rate determining oxidative alkyl-group transfer preceded by an unexpected C−H activation route on the Pd center during the catalytic cycle, where the deprotonation is assisted by an external triflate anion.
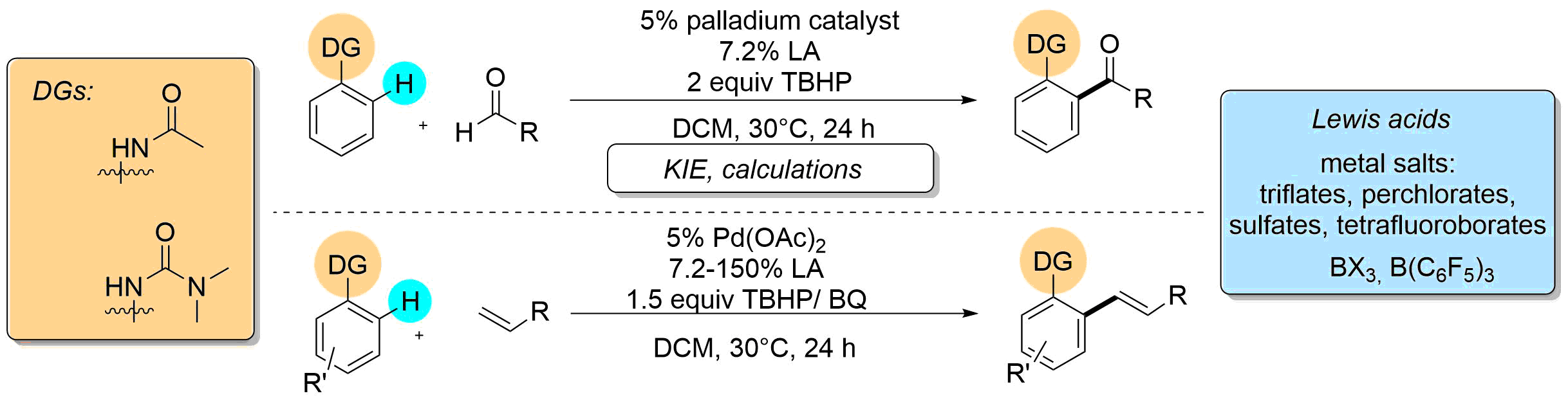
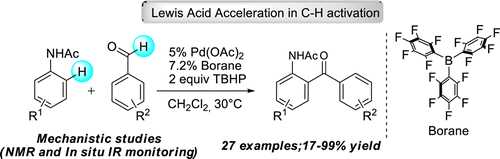
56. Study of Lewis Acid Accelerated Palladium Catalyzed C-H Activation
Study of Lewis Acid Accelerated Palladium Catalyzed C-H Activation, Orsolya Tischler, Szabolcs Kovács, Gábor Érsek, Péter Králl, János Daru, András Stirling, Zoltán Novák, J. Mol. Catal. Chem. 2017, 426, 444-450. DOI: 10.1016/j.molcata.2016.09.018
This paper is dedicated to Professor Georgiy B. Shul’pin on the occasion of his 70th birthday.
Keywords
C-H activation; palladium; Lewis acid; DFT studies
Highlights
•Amide and urea directed ortho-acylation and ortho-olefination on aromatic cores is accelerated by Lewis acidic additives.
•Lewis acidic additives afford more electrophilic transition metal center, favoring C-H activation.
•The efficiency of several palladium(II) catalysts is raised.
•KIE experiments and calculations suggests a rate-determining C-H activation step.
Abstract
Acceleration of palladium catalyzed C-H activation by various Lewis Acids was demonstrated on the directed ortho-alkenylation and acylation of acetanilide and urea derivatives. The universality of this effect was investigated by the study of different palladium catalysts, directing groups in the aromatic substrates and versatile Lewis acids. Experiments were carried out to monitor the reactions and to compare the behavior and activity of different types of Lewis acids. Kinetic investigation revealed a rate determining C-H activation step, and DFT studies were performed for the explanation of Lewis acid effect on C-H activation.
55. Mild Palladium Catalyzed ortho C-H Bond Functionalizations of Aniline Derivatives
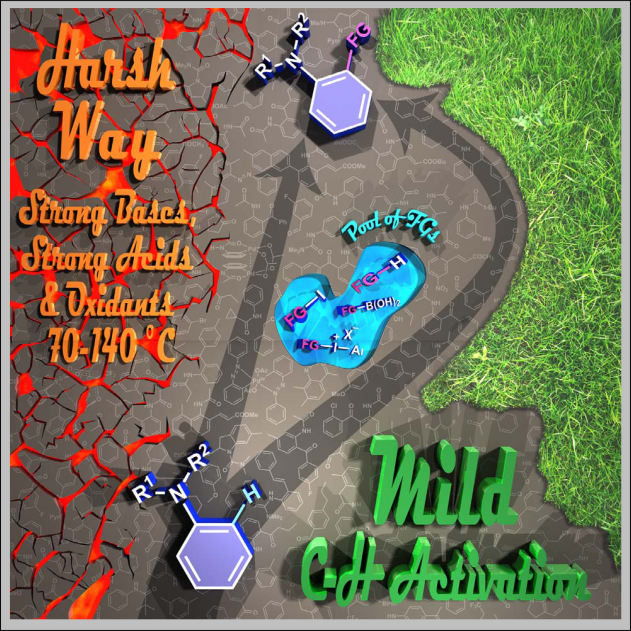
Mild Palladium Catalyzed ortho C-H Bond Functionalizations of Aniline Derivatives, Orsolya Tischler, Balázs L. Tóth, Zoltán Novák, Chem. Rec. 2017, 17, 184-199. DOI: 10.1002/tcr.201600059 | [Full Text Link]
This account collects the developments and transformations which avoid the utilization of harsh reaction conditions in the field of palladium catalyzed, ortho-directed C-H activation of aniline derivatives from the first attempts to up-to-date results, including the results of our research laboratory. The discussed functionalizations performed under mild conditions include acylation, olefination, arylation, alkylation, alkoxylation reactions. Beside the optimization studies and the synthetic applications mechanistic investigations are also presented.
54. Continuous flow synthesis of heterocyclic scaffolds Design principles of multistep systems – A review
Continuous flow synthesis of heterocyclic scaffolds. Design principles of multistep systems – A review, Klára Lövei, Péter Bana, Róbert Örkényi, György I. Túrós, János Éles, Zoltán Novák, Ferenc Faigl, Chim. Oggi Chem. Today, 2016, 34, 18-21. Link
KEYWORDS: Continuous flow synthesis, condensed heterocycles, scaffold, multistep flow synthesis, telescoping.
ABSTRACT: The synthesis of novel heterocycles is an essential task in small-molecule drug discovery. Continuous flow processing opens the way for a new paradigm in laboratory-scale synthesis as well as pharmaceutical manufacturing. Based on our experiences with the multistep synthesis of condensed benzothiazoles, we gathered some of the key design features in light of literature examples.
53. Recent advances in dual transition metal–visible light photoredox catalysis
Recent advances in dual transition metal–visible light photoredox catalysis, Balázs L. Tóth, Orsolya Tischler, Zoltán Novák, Tetrahedron Lett. 2016, 57, 4505–4513. DOI: 10.1016/j.tetlet.2016.08.081 |
In this Digest Letter, we collected and shortly summarized the recently developed dual transition metal–visible light photoredox catalytic processes including arylation, alkynylation, alkenylation, allylation, alkylation, fluoroalkylation, benzylation, acylation, and cyclization reactions. The utilization of multimetallic catalytic systems provides new synthetic strategies for the synthesis and functionalization of versatile and novel organic compounds.
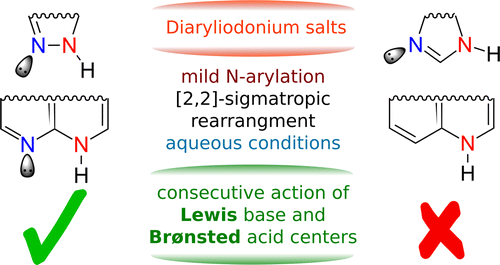
52. Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N‑Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study
Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N‑Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study, Tamás Bihari, Bence Babinszki, Zsombor Gonda, Szabolcs Kovács, Zoltán Novák, András Stirling, J. Org. Chem. 2016, 81, 5417-5422. DOI: 10.1021/acs.joc.6b00779 | [Full Text Link] [Supp. Info. Link]
The mechanism of arylation of N-heterocycles with unsymmetric diaryliodonium salts is elucidated. The fast and efficient N-arylation reaction is interpreted in terms of the bifunctionality of the substrate: The consecutive actions of properly oriented Lewis base and Brønsted acid centers in sufficient proximity result in the fast and efficient N-arylation. The mechanistic picture points to a promising synthetic strategy where suitably positioned nucleophilic and acidic centers enable functionalization, and it is tested experimentally.
51. Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies
Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies, Klára Aradi, Balázs L. Tóth, Gergely L. Tolnai, Zoltán Novák, Synlett 2016, 27, 1456-1485. DOI: 10.1055/s-0035-1561369 | [Full Text Link]
Keywords: arylation - diaryliodonium salts - hypervalent iodine reagents - metal-free - copper-catalyzed - palladium-catalyzed - C–H activation - direct functionalization
This account aims to give a description of the usefulness of diaryliodonium salts in organic chemistry, including their synthesis and applications in the presence and absence of transition-metal catalysts. Herein, we briefly summarize the structural properties and reactivity of diaryliodonium salts. We describe several applications of these hypervalent reagents including metal-free arylations of C-, O-, N- and S-nucleophiles. The synthesis and functionalization of aromatic and heteroaromatic systems via copper- and palladium-catalyzed transformations are also discussed in this account.
Content:
1 Introduction
2 Structural Properties and Reactivity
3 Applications of Diaryliodonium Salts in Organic Syntheses
3.1 Transition-Metal-Free Arylations of Heteroatom and Carbon Nucleophiles
3.1.1 Arylation of Oxygen Nucleophiles
3.1.1.1 Arylation of (Hetero)aromatic Alcohols
3.1.1.2 Arylation of Aliphatic Alcohols
3.1.1.3 Arylation of Acids
3.1.2 Arylation of Nitrogen Nucleophiles
3.1.2.1 N-Arylation of Amines and Amides
3.1.2.2 N-Arylation of Heterocycles
3.1.3 Arylation of Sulfur Nucleophiles
3.1.4 Arylation of Inorganic Anions
3.1.5 Arylation of Carbon Nucleophiles
3.1.5.1 Electron-Rich Heterocycles
3.1.5.2 Arylation of Carbonyl and Nitro Compounds
3.2 Synthesis and Functionalization of Aromatic and Heteroaromatic Molecules with Diaryliodonium Salts in the Presence of Copper and Palladium Catalysts
3.2.1 C–H Functionalizations by Copper-Catalyzed C–H Arylations
3.2.2 Copper-Catalyzed Cyclization of Unsaturated Compounds with Diaryliodonium Salts
3.2.3 Palladium-Catalyzed Arylations
3.2.3.1 Electrophilic Palladation and Arylation
3.2.3.2 Directing-Group-Assisted C–H Bond Activation and Arylation
4 Conclusion
50. Activation of C–H Activation: The Beneficial Effect of Catalytic Amount of Triaryl Boranes on Palladium-Catalyzed C–H Activation
Activation of C–H Activation: The Beneficial Effect of Catalytic Amount of Triaryl Boranes on Palladium-Catalyzed C–H Activation, Orsolya Tischler, Zsófia Bokányi, Zoltán Novák, Organometallics 2016, 35, 741-746. DOI: 10.1021/acs.organomet.5b01017 | [Full Text Link] [Supp. Info. Link]
Herein we report a novel approach to the acceleration of palladium-catalyzed C–H activation reactions. We demonstrated that the utilization of electron-deficient triaryl boranes as Lewis acidic cocatalysts of palladium enables the directed cross dehydrogenative coupling of aldehydes and anilides under mild reaction conditions. Study of the kinetic profile of the transformation reveals a unique, unexpectedly long induction period of the transformation.
49. Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C-H Activation
Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C-H Activation, Balázs L. Tóth, Szabolcs Kovács, Gergő Sályi, Zoltán Novák, Angew. Chem. Int. Ed. 2016, 55, 1988-1992. DOI: 10.1002/anie.201510555 | [Full Text Link] [Supp. Info. Link]
The introduction of trifluoroalkyl groups into aromatic molecules is an important transformation in the field of organic and medicinal chemistry. However, the direct installation of fluoroalkyl groups onto aromatic molecules still represents a challenging and highly demanding synthetic task. Herein, a simple trifluoroethylation process that relies on the palladium-catalyzed C-H activation of aromatic compounds is described. With the utilization of a highly active trifluoroethyl(mesityl)iodonium salt, the developed catalytic method enables the first highly efficient and selective trifluoroethylation of aromatic compounds. The robust catalytic procedure provides the desired products in up to 95 % yield at 25 °C in 1.5 to 3 hours and tolerates a broad range of functional groups. The utilization of hypervalent reagents opens new synthetic possibilities for direct alkylations and fluoroalkylations in the field of transition-metal-catalyzed C-H activation.
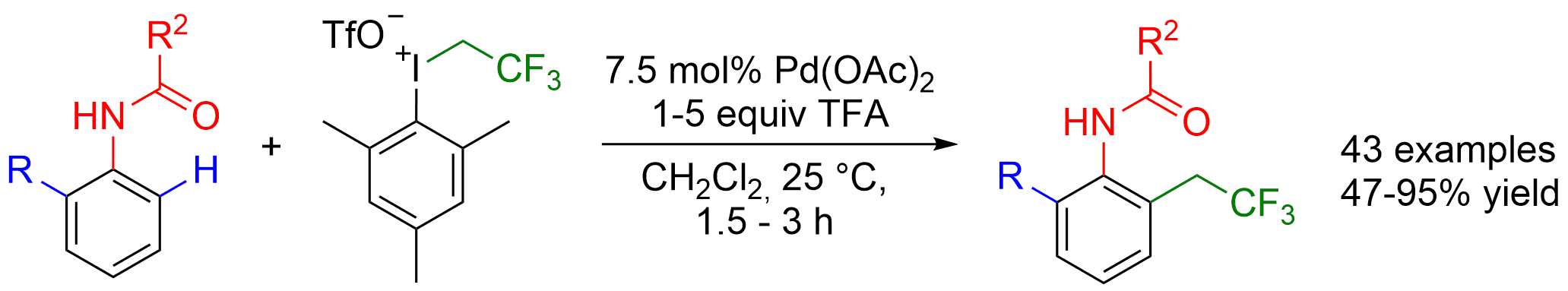
Simple: Anilides can be trifluoroethylated with a 2,2,2-trifluoroethyl-substituted iodonium salt in the presence of a palladium catalyst under mild conditions (see scheme). The reaction proceeds by CH activation at the ortho position and features a broad substrate scope.
Frontispiece: Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C−H Activation, DOI: 10.1002/anie.201680661
Trifluoroethylation In their Communication on page 1988 ff., Z. Novák and co-workers describe the use of a trifluoroethyl(mesityl)iodonium salt for the simple and efficient palladium-catalyzed trifluoroethylation of aromatic compounds by C−H activation.
48. Modular Copper-Catalyzed Synthesis of Chromeno[4,3-b]quinolines with the Utilization of Diaryliodonium Salts
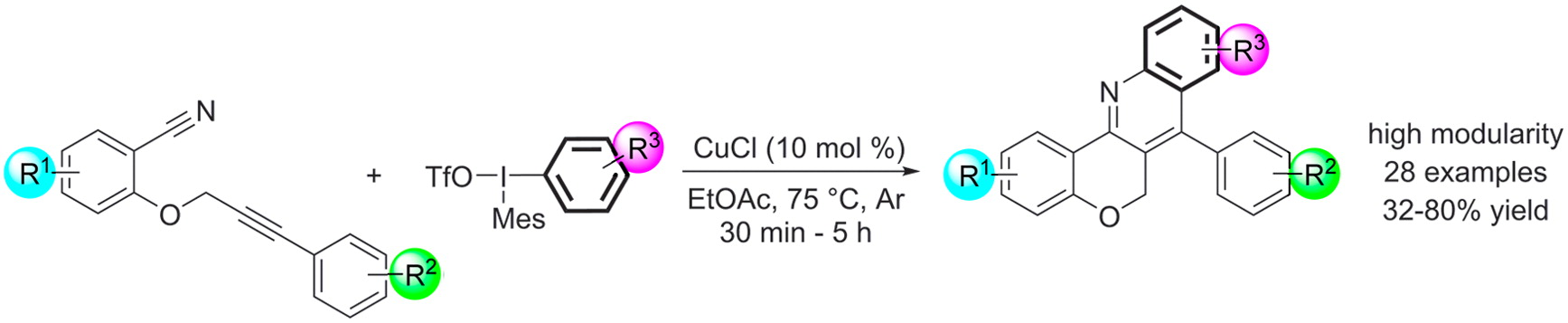
Modular Copper-Catalyzed Synthesis of Chromeno[4,3-b]quinolines with the Utilization of Diaryliodonium Salts, Klára Aradi, Petra Bombicz, Zoltán Novák, J. Org. Chem. 2016, 81, 920-931. DOI: 10.1021/acs.joc.5b02490 | [Full Text Link] [Supp. Info. Link, CIF]
A novel, highly modular synthetic method with high functional group tolerance was developed for the construction of chromenoquinoline derivatives from arylpropynyloxy-benzonitriles and diaryliodonium triflates via an oxidative arylation–cyclization path. The copper(I) chloride catalyzed reaction is presumed to involve the formation of highly active arylcopper(III) species.
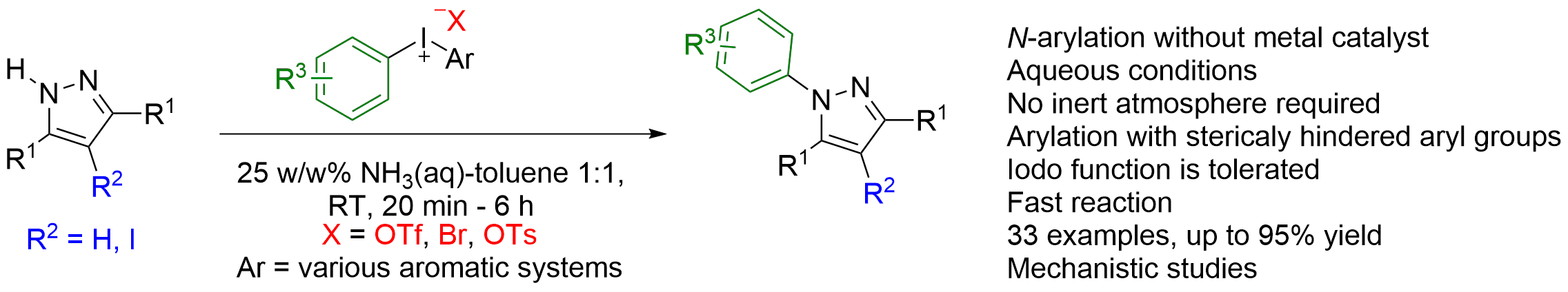
47. Transition-Metal-Free N-Arylation of Pyrazoles with Diaryliodonium Salts
Transition-Metal-Free N-Arylation of Pyrazoles with Diaryliodonium Salts, Zsombor Gonda, Zoltán Novák, Chem. Eur. J. 2015, 21, 16801-16806. DOI: 10.1002/chem.201502995 | [Full Text Link] [Supp. Info. Link]
A new synthetic method was developed for the N-arylation of pyrazoles using diaryliodonium salts. The transformation does not require any transition-metal catalyst and provides the desired N-arylpyrazoles rapidly under mild reaction condition in the presence of aqueous ammonia solution as a mild base without the use of inert atmosphere. The chemoselectivity of unsymmetric diaryliodonium salts was also explored with large number of examples.
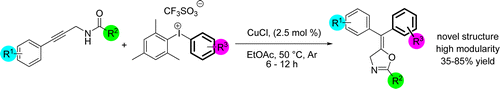
46. Utilization of Copper-Catalyzed Carboarylation–Ring Closure for the Synthesis of New Oxazoline Derivatives
Utilization of Copper-Catalyzed Carboarylation–Ring Closure for the Synthesis of New Oxazoline Derivatives, Ádám Sinai, Dóra Vangel, Tamás Gáti, Petra Bombicz, Zoltán Novák, Org. Lett. 2015, 17, 4136-4139. DOI: 10.1021/acs.orglett.5b01860 | [Full Text Link] [Supp. Info. Link, CIF]
A copper-catalyzed carboarylation–ring-closure strategy was used for the modular synthesis of oxazolines via the reaction of 1-aryl- and 1-alkylpropargylamides and diaryliodonium salts. The novel approach enables the efficient, modular synthesis of oxazoline derivatives bearing fully substituted exo double bonds.
45. Multistep Continuous-Flow Synthesis of Condensed Benzothiazoles
Multistep Continuous-Flow Synthesis of Condensed Benzothiazoles, Klára Lövei, István Greiner, János Éles, Áron Szigetvári, Miklós Dékány, Sándor Lévai, Zoltán Novák, György István Túrós, J. Flow Chem. 2015, 5, 74–81. DOI: 10.1556/1846.2015.00004 | [Full Text Link] [Supp. Info Link]
In medicinal chemistry, the development of synthetic procedures for the access of new heterocyclic systems as potential scaffolds is elementary. Herein, we report our results on the formation of small drug-like heterocycles, utilizing flow chemistry. This approach enables the extension of the reaction parameter window, including high-pressure/high-temperature or hazardous chemistry. In our work, various novel condensed tricyclic benzothiazoles fused with furo- and thieno-rings were synthesized applying a multistep continuous-flow protocol. The process includes two ring closure steps and a nitro group reduction step. Batch and telescoped continuous-flow syntheses were also designed and performed.
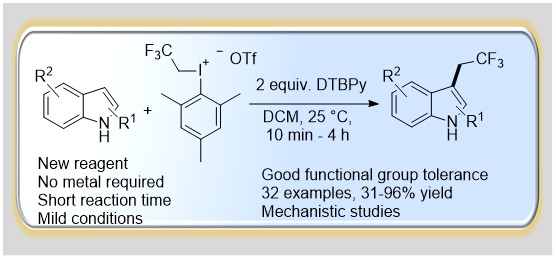
44. Efficient Direct 2,2,2-Trifluoroethylation of Indoles via C-H Functionalization
Efficient Direct 2,2,2-Trifluoroethylation of Indoles via C-H Functionalization, Gergely László Tolnai, Anna Székely, Zita Makó, Tamás Gáti, János Daru, Tamás Bihari, Andras Stirling and Zoltán Novák, Chem. Commun. 2015, 51, 4488-4491. DOI: 10.1039/C5CC00519A | [Supp. Info Link]
A novel highly C3 selective metal free trifluoroethylation of indoles using 2,2,2-trifuoroethyl(mesityl)-iodonium triflate was developed. The methodology enables the introduction of a trifluoroethyl group in a fast and efficient reaction under mild conditions with high functional group tolerance. Beyond the synthetic developments, quantum chemical calculations provide a deeper understanding of the transformation.
43. Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines
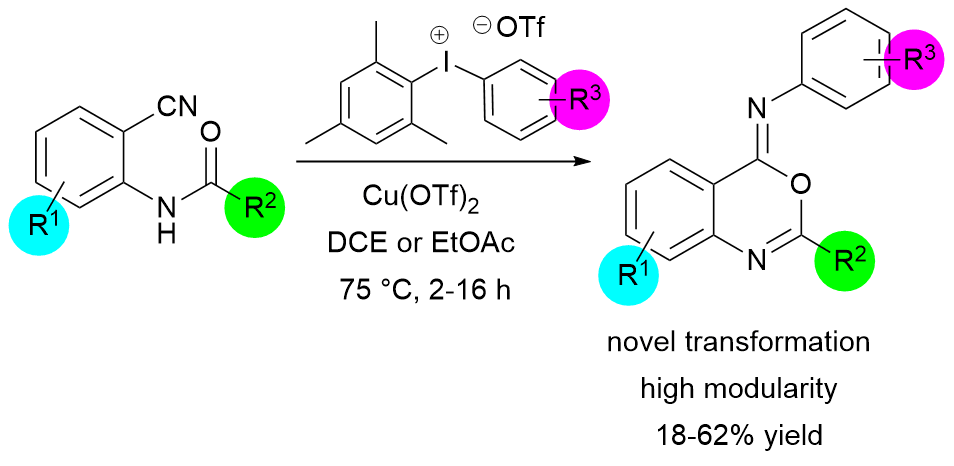
Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines, Klára Aradi, Zoltán Novák, Adv. Synt. Catal. 2015, 357, 371-376. DOI: 10.1002/adsc.201400763 | [Full Text Link] [Supp. Info Link]
A novel, highly modular synthetic methodology with high functional group tolerance was developed for the construction of iminobenzoxazine derivatives from ortho-cyanoanilides and diaryliodonium triflates via an oxidative arylation–cyclization path. The reaction is supposed to involve the formation of highly active aryl-copper(III) species. In this novel transformation, copper(II) triflate was used as catalyst in 1,2-dichloroethane or ethyl acetate and the reaction takes place at 75 °C in 2–16 h.
42. Synthesis and Transformations of Oxygen Heterocycles
Synthesis and Transformations of Oxygen Heterocycles, Zoltán Novák, András Kotschy, Topics in Heterocyclic Chemistry Vol. 45, 231-303. DOI: 10.1007/7081_2014_136
The recent developments in the transition metal-catalyzed synthesis and transformations of such oxygen-containing heteroaromatic systems are reviewed, where the oxygen is part of a five-membered ring.
41. Mechanistic Study of Silver-Mediated Furan Formation by Oxidative Coupling
Mechanistic Study of Silver-Mediated Furan Formation by Oxidative Coupling, János Daru, Zsuzsanna Benda, Ádám Póti, Zoltán Novák, András Stirling, Chem. Eur. J. 2014, 20, 15395–15400. DOI: 10.1002/chem.201404302 | [Full Text Link] [Supp. Info Link]
Density functional calculations and experiments have been carried out to unravel the mechanism of a silver-mediated furan formation by oxidative coupling. Various possible reaction paths were considered and the most favorable channel has been identified on the basis of the calculated solvent-corrected Gibbs free-energy profiles. The mechanism represented by this route consists of a radical and a subsequent ionic route. The silver cation has a double role in the mechanism: it is the oxidant in the radical steps and the catalyst for the ionic steps, which is in accordance with the experimental observations. The two most important aspects of the optimal route are the formation of a silver–acetylide, reacting subsequently with the enolate radical, and the aromatic furan-ring formation in a single step at the latter, ionic segment of the reaction path. Our findings could explain several experimental observations, including the “key-promoter role” of silver, the preference for ionic cyclization, and the reduced reactivity of internal acetylides.
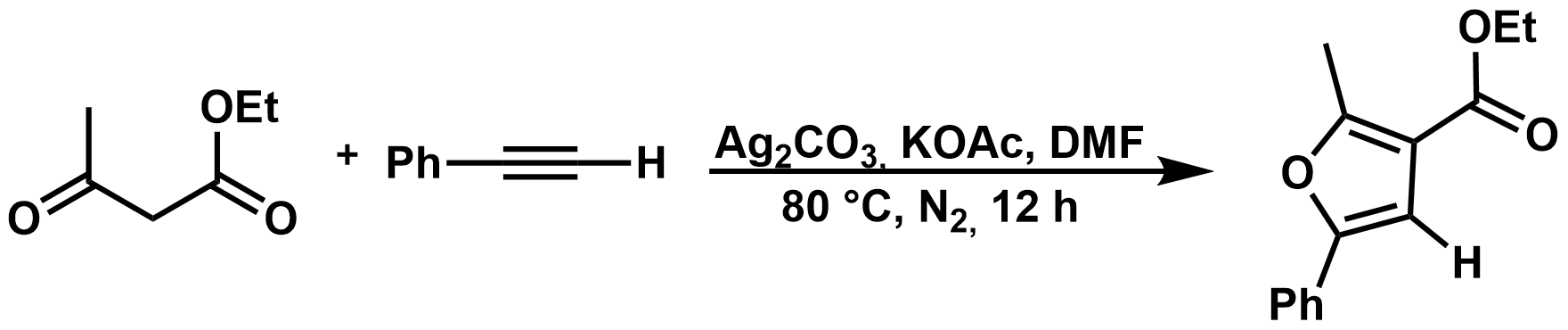
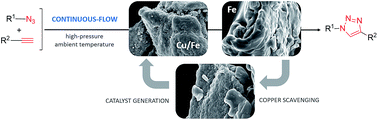
40. Continuous-flow azide–alkyne cycloadditions with an effective bimetallic catalyst and a simple scavenger system
Continuous-flow azide–alkyne cycloadditions with an effective bimetallic catalyst and a simple scavenger system, Sándor B. Ötvös, Gábor Hatoss, Ádám Georgiádes, Szabolcs Kovács, István M. Mándity, Zoltán Novák, Ferenc Fülöp, RSC Adv. 2014, 4, 46666-46674. DOI: 10.1039/C4RA07954J | [Supp. Info Link]
A flow chemistry-based technique is presented herein for Cu(I)-catalyzed azide–alkyne cycloadditions with a copper on iron bimetallic system as the catalyst and iron powder as a readily available copper scavenger. The method proved to be rapid and safe as compared with the conventional batch experiment; and by using an in-line copper scavenger, the level of copper impurities in the triazole products could readily be reduced to negligibly small amounts. The process was widely applicable, as not only terminal alkynes, but also various disubstituted acetylenes were nicely tolerated as dipolarophiles leading to useful 1,4,5-trisubstituted 1,2,3-triazoles.
39. Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and Heteroaromatic Iodides: The Beneficial Anchoring Effect of Borates
Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and Heteroaromatic Iodides: The Beneficial Anchoring Effect of Borates, Zsombor Gonda, Szabolcs Kovács, Csaba Wéber, Tamás Gáti, Attila Mészáros, András Kotschy, Zoltán Novák, Org. Lett. 2014, 16, 4268-4271. DOI: 10.1021/ol501967c | [Full Text Info] [Supp. Info Link, NMR]
Efficient copper-catalyzed trifluoromethylation of aromatic iodides was achieved with TMSCF3 in the presence of trimethylborate. The Lewis acid was used to anchor the in situ generated trifluoromethyl anion and suppress its rapid decomposition. Broad applicability of the new trifluoromethylating reaction was demonstrated in the functionalization of different aromatic and heteroaromatic iodides.
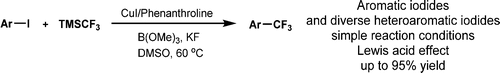
38. Utilization of a Copper on Iron Catalyst for the Synthesis of Biaryl Systems and Benzoxazines via Oxidative Arylation of Anilide Derivatives
Utilization of a Copper on Iron Catalyst for the Synthesis of Biaryl Systems and Benzoxazines via Oxidative Arylation of Anilide Derivatives, Anna Székely, Ádám Sinai, Edina B. Tóth, Zoltán Novák, Synthesis 2014, 14, 1871-1880. DOI: 10.1055/s-0033-1338642 | [Full Text Link] [Supp. Info Link]
Heterogeneous copper on iron catalyst serves as an efficient alternative copper source for arylation reactions using hypervalent iodonium salts. The copper(0) catalyst affords meta-arylation of pivalanilides, while 2-ethynylanilides undergo oxidative carboarylation–ring closure with diaryliodonium salts.
37. Palladium-Catalyzed Methoxylation of Aromatic Chlorides with Borate Salts
Palladium-Catalyzed Methoxylation of Aromatic Chlorides with Borate Salts, Gergely L. Tolnai, Bálint Pethő, Péter Králl, Zoltán Novák, Adv. Synth. Catal. 2014, 356, 125-129. DOI: 10.1002/adsc.201300687 | [Full Text Link] [Supp. Info Link]
Herein we disclose a simple palladium-catalyzed transformation for the methoxylation of aromatic chlorides with tetramethoxyborate salts. The procedure provides a new and efficient synthetic tool for the introduction of a methoxy group into aromatic systems. In addition, the reaction can be achieved using a wide range of aromatic and heteroaromatic chlorides, the cheapest class of halides.
36. A one-pot process for palladium catalyzed direct C-H acylation of anilines in water using a removable ortho directing group
A one-pot process for palladium catalyzed direct C-H acylation of anilines in water using a removable ortho directing group ,Fruzsina Szabó, Dániel Simkó, Zoltán Novák, RSC Advances 2014, 4, 3883-3886. DOI: 10.1039/C3RA45160G | [Supp. Info Link]
A new mild, practical method for the synthesis of aminobenzophenone derivatives through a three step one-pot reaction sequence involving acylation of anilines, palladium catalyzed cross-dehydrogenative coupling of the formed anilides and the hydrolytic cleavage is reported. The full reaction sequence was performed under aqueous conditions.
35. Copper-Catalyzed Oxidative Ring Closure and Carboarylation of 2-Ethynylanilides
Copper-Catalyzed Oxidative Ring Closure and Carboarylation of 2-Ethynylanilides, Ádám Sinai, Ádám Mészáros, Tamás Gáti, Veronika Kudar , Anna Palló, Zoltán Novák, Org. Lett. 2013, 15, 5654–5657. DOI: 10.1021/ol402600r | [Full Text Link] [Supp. Info Link, CIF1, CIF2]
A new copper-catalyzed oxidative ring closure of ethynyl anilides with diaryliodonium salts was developed for the highly modular construction of benzoxazines bearing a fully substituted exo double bond. The oxidative transformation includes an unusual 6-exo-dig cyclization step with the formation of C–O and C–C bonds.
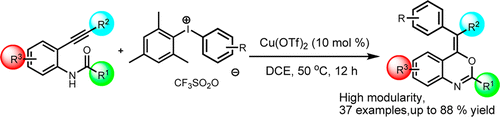
34. Copper on iron promoted one-pot synthesis of β-aminoenones and 3,5-disubstituted pyrazoles
Copper on iron promoted one-pot synthesis of β-aminoenones and 3,5-disubstituted pyrazoles, Szabolcs Kovács, Zoltán Novák, Tetrahedron 2013, 69, 8987–8993. DOI: 10.1016/j.tet.2013.08.047 |
The reaction of hydroximoyl chlorides with acetylenes in the presence of a copper on iron bimetallic system leads to β-aminoenones via reductive ring opening of isoxazole intermediates. The valuable β-aminoenone building blocks can be isolated or transformed into pyrazoles with the addition of hydrazine in a straightforward one-pot procedure.
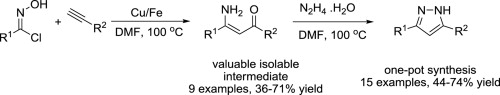
33. Mild Palladium-Catalyzed Oxidative Direct ortho C-H aczlation of Anilides under Aqueous Conditions
Mild Palladium-Catalyzed Oxidative Direct ortho C-H aczlation of Anilides under Aqueous Conditions, Fruzsina Szabó, János Daru, Dániel Simkó, Tibor Zs. Nagy, András Stirling, Zoltán Novák, Adv. Synth. Catal. 2013, 355, 685-691. DOI: 10.1002/adsc.201200948 | [Full Text Link] [Supp. Info Link]
Palladium-catalyzed cross-dehydrogenative coupling between anilides and aromatic aldehydes was achieved under aqueous conditions. A wide variety of the desired benzophenone derivatives was isolated in good to excellent yield. The reaction rate acceleration effect of acid and detergent has been demonstrated. Mechanistic insight has been obtained from quantum chemical calculations.
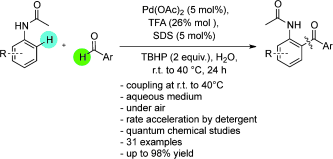
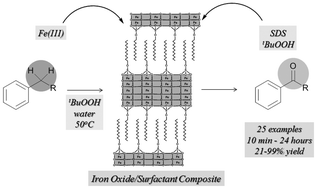
32. Iron-surfactant nanocomposite-catalyzed benzylic oxidation in water
Iron-surfactant nanocomposite-catalyzed benzylic oxidation in water, Fruzsina Szabó, Bálint Pethő, Zsombor Gonda, Zoltán Novák, RSC Advances 2013, 3, 4903-4908. DOI: 10.1039/C3RA22856H | [Supp. Info Link]
Benzylic oxidation in the presence of an iron–surfactant nanocomposite catalyst under aqueous conditions is described. A significant reaction rate acceleration in the presence of anionic surfactants was demonstrated. Several benzylic substrates were efficiently transformed to ketones under mild conditions.
31. Copper on Iron: Catalyst and Scavanger for Azide-Alkzne Cycloaddition
Copper on Iron: Catalyst and Scavanger for Azide-Alkzne Cycloaddition, Szabolcs Kovács, Katalin Zih-Perényi, Ádám Révész, Zoltán Novák, Synthesis 2012; 44, 3722-3730. DOI: 10.1055/s-0032-1317697 | [Full Text Link] [Supp. Info Link]
Dipolar cycloaddition of terminal alkynes and azides catalyzed by the Cu/Fe bimetallic system is reported. In the presence of a readily accessible nanosized copper source, the cycloaddition reaction can be easily achieved at ambient temperature with high efficiency. The product obtained from the reaction catalyzed by Cu/Fe contains significantly lower copper contaminants compared to various active homogeneous copper complexes. Iron not only behaves as support for copper, but acts as a redox scavenger, and reduces the copper contamination of the organic product.
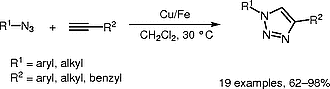
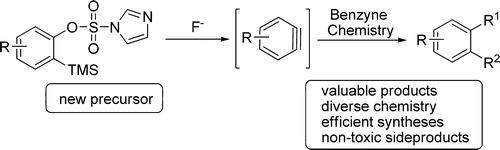
30. Design and Application of New Imidazolylsulfonate-Based Benzyne Precursor: An Efficient Triflate Alternative
Design and Application of New Imidazolylsulfonate-Based Benzyne Precursor: An Efficient Triflate Alternative, Szabolcs Kovács, Ádám I. Csincsi, Tibor Zs. Nagy, Sándor Boros, Géza Timári, Zoltán Novák, Org. Lett. 2012, 14, 2022-2025. DOI: 10.1021/ol300529j | [Full Text Link] [Supp. Info Link]
Several o-(trimethylsilyl)aryl imidazolylsulfonates were synthesized in a simple process and successfully applied in cycloadditions involving benzyne intermediates. The precursor offers an efficient alternative for generating benzynes compared to widely used ortho TMS triflates under similar reaction conditions. With the utilization of this new precursor, the formation of potentially genotoxic trifluoromethanesulfonate side product is eliminated. The applicability of the new benzyne precursor was demonstrated in different types of cycloaddition reactions to prepare heterocyclic molecules.
29. Evaluation of bis-triphenylphosphano-copper(I)-butyrate (C3H7COOCu(PPh3)2) as catalyst for the synthesis of 1-(D-glycopyranosyl)-4-substituted-1,2,3-triazoles
Evaluation of bis-triphenylphosphano-copper(I)-butyrate (C3H7COOCu(PPh3)2) as catalyst for the synthesis of 1-(D-glycopyranosyl)-4-substituted-1,2,3-triazoles, Éva Bokor, Csenge Koppány, Zsombor Gonda, Zoltán Novák, László Somsák, Carbohydr. Res. 2012, 351, 42-48. DOI: 10.1016/j.carres.2012.01.004 | [Full Text Link]
Bis-triphenylphosphano-copper(I)-butyrate (C3H7COOCu(PPh3)2) was applied for the synthesis of O-peracylated 1-glycopyranosyl-4-substituted-1,2,3-triazoles from the corresponding glycosyl azides and alkynes. This catalyst proved superior to the CuSO4/l-ascorbic acid system even with sterically hindered and less reactive glycosyl azides.
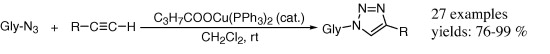
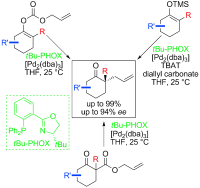
28. Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies
Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies, Douglas C. Behenna, Justin T. Mohr,Nathaniel H. Sherden, Smaranda C. Marinescu, Andrew M. Harned, Kousuke Tani, Masaki Seto, Sandy Ma, Zoltán Novák, Michael R. Krout, Ryan M. McFadden, Jennifer L. Roizen, John A. Enquist Jr., David E. White, Samantha R. Levine, Krastina V. Petrova, Akihiko Iwashita, Scott C. Virgil, Brian M. Stoltz, Chem. Eur. J. 2011, 17, 14199-14223. DOI: 10.1002/chem.201003383 | [Full Text Link] [Supp. Info Link]
α-Quaternary ketones are accessed through novel enantioselective alkylations of allyl and propargyl electrophiles by unstabilized prochiral enolate nucleophiles in the presence of palladium complexes with various phosphinooxazoline (PHOX) ligands. Excellent yields and high enantiomeric excesses are obtained from three classes of enolate precursor: enol carbonates, enol silanes, and racemic β-ketoesters. Each of these substrate classes functions with nearly identical efficiency in terms of yield and enantioselectivity. Catalyst discovery and development, the optimization of reaction conditions, the exploration of reaction scope, and applications in target-directed synthesis are reported. Experimental observations suggest that these alkylation reactions occur through an unusual inner-sphere mechanism involving binding of the prochiral enolate nucleophile directly to the palladium center.
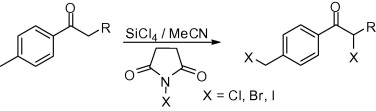
27. N-Halosuccinimide/SiCl4 as general, mild and efficient systems for the α-monohalogenation of carbonyl compounds and for benzylic halogenation
N-Halosuccinimide/SiCl4 as general, mild and efficient systems for the α-monohalogenation of carbonyl compounds and for benzylic halogenation, Tarek A. Salama, Zoltán Novák, Tetrahedron Lett. 2011, 52, 4026-4029. DOI: 10.1016/j.tetlet.2011.05.135 |
Combinations of N-halosuccinimide and tetrachlorosilane in acetonitrile were found to be efficient systems for the selective α-monohalogenation of carbonyl compounds as well as for benzylic halogenation under mild conditions.
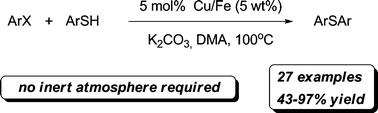
26. Oxidoreductive Coupling of Thiols with Aryl Halides Catalyzed by Copper on Iron
Oxidoreductive Coupling of Thiols with Aryl Halides Catalyzed by Copper on Iron, Szabolcs Kovács, Zoltán Novák, Org. Biomol. Chem. 2011, 9, 711-716. DOI: 10.1039/C0OB00397B | [Supp. Info Link]
Synthesis and utilization of a simple copper on iron catalyst in the coupling of aryl halides with thiols through disulfide intermediate is reported. The iron support of copper catalyst ensures reductive media for the coupling, allows easy removal of the metals by outer magnetic field and enables the recycling of the catalyst.
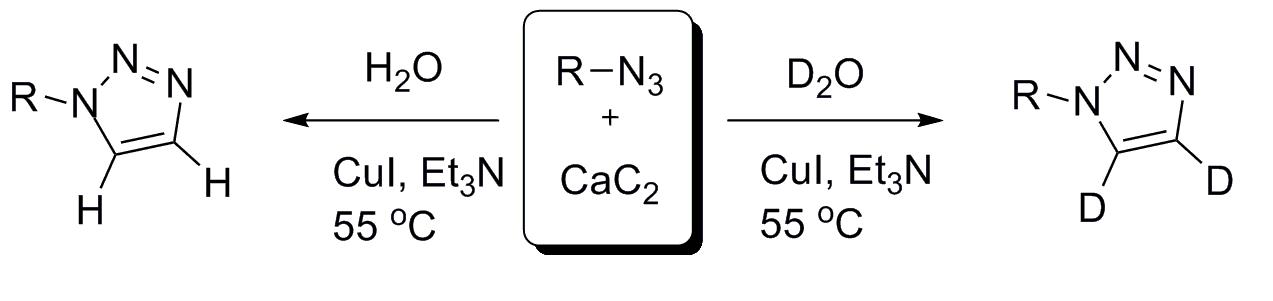
25. Efficient Synthesis of Deuterated 1,2,3-Triazoles
Efficient Synthesis of Deuterated 1,2,3-Triazoles, Zsombor Gonda, Krisztán Lőrincz, Zoltán Novák, Tetrahedron Lett. 2010, 51, 6275-6277. DOI: 10.1016/j.tetlet.2010.09.097 | [Full Text Link]
A wide variety of 1-monosubstituted 1,2,3-triazoles were synthesized efficiently via a copper-catalyzed click-reaction between azides and acetylene gas, generated in situ from CaC2 with the addition of H2O or D2O.
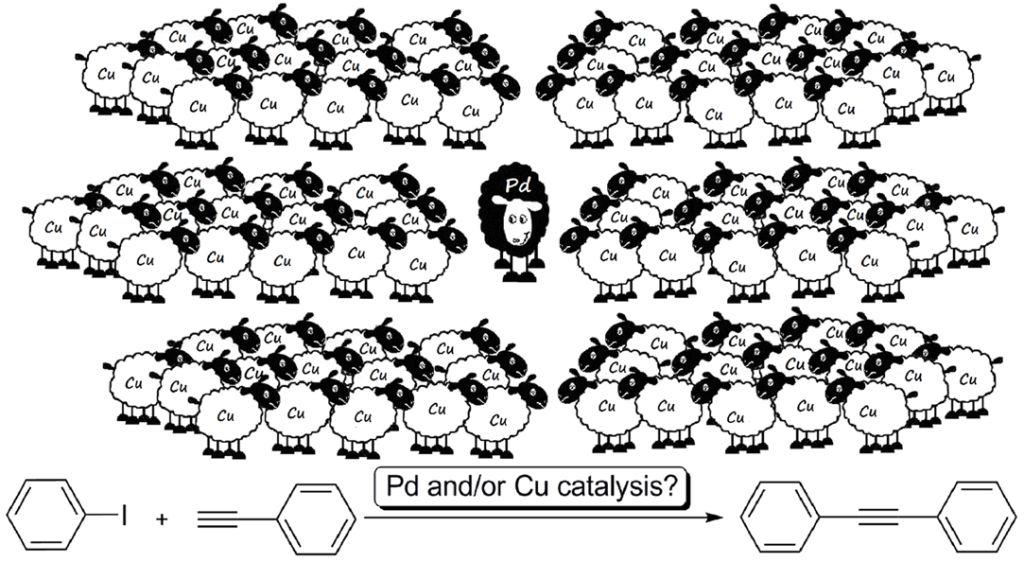
24. Dramatic Impact of ppb Levels of Palladium on the "Copper-Catalyzed" Sonogashira Coupling
Dramatic Impact of ppb Levels of Palladium on the "Copper-Catalyzed" Sonogashira Coupling, Zsombor Gonda, Gergely L. Tolnai, Zoltán Novák, Chem. Eur. J. 2010, 16, 11822-11826. DOI: 10.1002/chem.201001880 | [Full Text Link] [Supp. Info Link]
Palladacadabra! The effect of ppb levels of palladium on the “copper-catalyzed” Sonogashira coupling is reported. The observed high sensitivity to palladium impurities queries the existence of pure copper catalysis in the coupling of aryl iodides and terminal acetylenes (see figure).
23. Activity of palladium on charcoal catalysts in cross-coupling reactions
Activity of palladium on charcoal catalysts in cross-coupling reactions, Anna Komáromi, Fruzsina Szabó, Zoltán Novák, Tetrahedron Lett. 2010, 51, 5411-5414. DOI: 10.1016/j.tetlet.2010.07.170 |
Comparison of the activity of several commercially available Pd/C catalysts in C–C, C–N, and C–S bond forming cross-coupling reactions has demonstrated the importance of the choice of the catalyst source. Investigations showed marked difference in activity between the catalysts. Moreover, the catalytic activity of each catalyst varies with respect to the coupling. The first Pd/C catalyzed Hiyama coupling is reported.
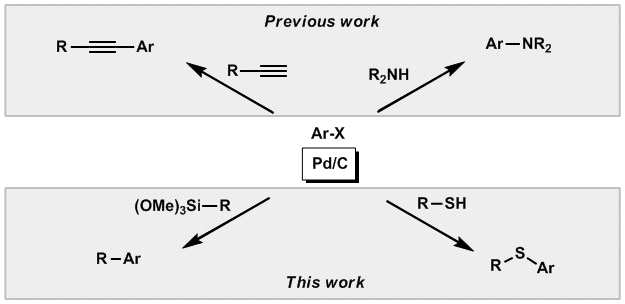
22. Examination of the Aromatic Amination Catalyzed by Palladium on Charcoal
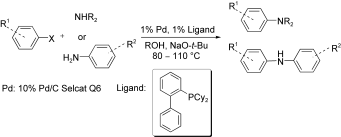
Examination of the Aromatic Amination Catalyzed by Palladium on Charcoal, Anna Komáromi, Zoltán Novák, Adv. Synth. Catal. 2010, 352, 1523. DOI: 10.1002/adsc.201000048 | [Full Text Link] [Supp. Info Link]
The Buchwald–Hartwig amination of aryl halides with secondary amines and functionalized aromatic amines catalyzed by solid-supported palladium is reported. The choices of ligand, base and solvent are crucial for the successful coupling. The amination of aromatic iodides, bromides and chlorides can be easily achieved with palladium on charcoal in the presence of a biphenylphosphane-type ligand at 80–110 °C. In addition, the palladium on charcoal catalyst is easily separable after the reaction, and reusable several times with only small activity loss.
21. Highly Active Copper Catalysts for Azide-Alkyne Cycloaddition
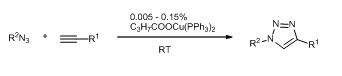
Highly Active Copper Catalysts for Azide-Alkyne Cycloaddition, Zsombor Gonda, Zoltán Novák, Dalton Trans. 2010, 39, 726-729. DOI: 10.1039/b920790m | [Supp. Info Link]
Bis-triphenylphosphano complexes of copper(I)-carboxylates serve as efficient catalysts for azide-alkyne cycloaddition. The triazole formation takes place straightforwardly at ambient temperature providing a wide variety of products with good yields in the presence of 0.005–0.05% catalyst.
20. The Sequential Sonogashira-Click Reaction: A Versatile Route to 4-Aryl-1,2,3-triazoles
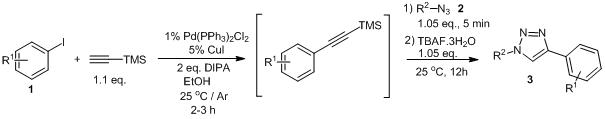
The Sequential Sonogashira-Click Reaction: A Versatile Route to 4-Aryl-1,2,3-triazoles, Krisztián Lőrincz, Péter Kele, Zoltán Novák, Synthesis 2009, 20, 3527-3532. DOI: 10.1055/s-0029-1216985 | [Full Text Link]
Aryl halides can be easily transformed in a one-pot procedure into 4-aryl-1,2,3-triazoles with palladium/copper-catalyzed Sonogashira-click reaction sequence, using trimethylsilylacetylene as acetylene surrogate.
19. Copper-free Sonogashira coupling in amine-water solvent mixtures
Copper-free Sonogashira coupling in amine-water solvent mixtures, Anna Komáromi, Gergely L. Tolnai, Zoltán Novák, Tetrahedron Lett. 2008, 49, 7294-7298. DOI: 10.1016/j.tetlet.2008.10.037 |
Extensive study of different amine–water solvent mixtures was carried out for copper free Sonogashira coupling of aryl iodides. The influence of the palladium sources, ligands, amine–water ratio and further additives was also evaluated. Application of sec-butylamine–water mixture proved to be an excellent medium for rapid and efficient coupling of aryl-iodides at ambient temperature in the presence of PdCl2(PPh3)2 as catalyst.
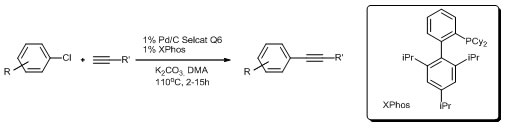
18. Efficient Copper-free Sonogashira Coupling of Aryl Chlorides with Palladium on Charcoal
Efficient Copper-free Sonogashira Coupling of Aryl Chlorides with Palladium on Charcoal, Anna Komáromi, Zoltán Novák, Chem. Commun. 2008, 4968-4970. DOI: 10.1039/b810928a | [Supp. Info Link]
Palladium on charcoal serves as an efficient and reusable solid supported catalyst for the Sonogashira coupling of aryl chlorides with terminal acetylenes in the presence of a bulky, electron-rich biphenyl type ligand (XPhos), without copper co-catalyst.