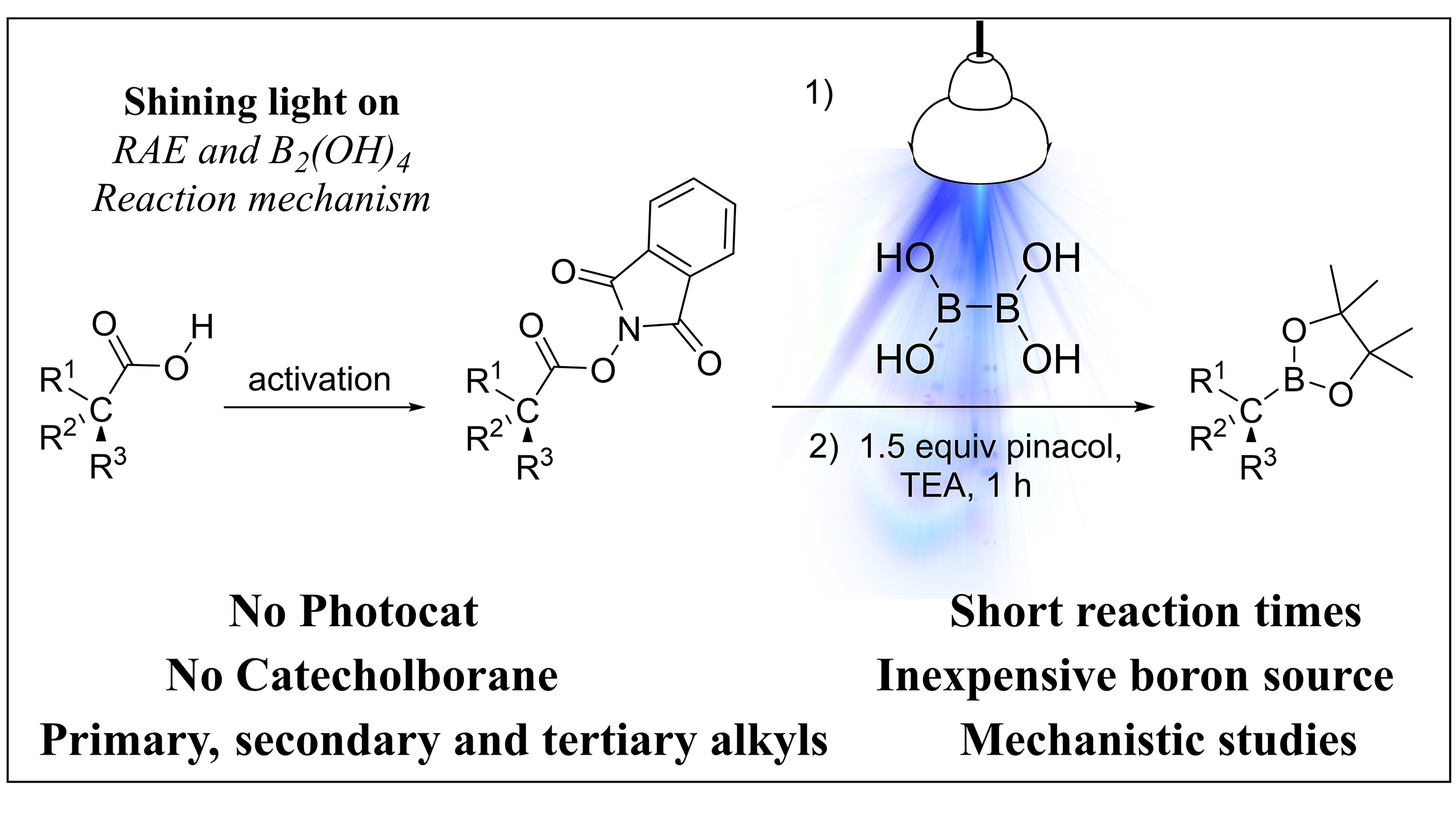
92. Iodonium based regioselective double nucleophilic alkene functionalization of a hydrofluoroolefin scaffold
Iodonium based regioselective double nucleophilic alkene functionalization of a hydrofluoroolefin scaffold, János T. Csenki, Zoltán Novák, Chem. Commun., 2024, 60, 726-729. DOI: 10.1039/D3CC04985J | [Full Text Link] [Supp. Info Link]
Herein, we report a modular regioselective alkene difunctionalization strategy based on the use of hydrofluoroolefin (HFO) gas as fluorous feedstock material. The transformation of the HFO gas to iodonium salt creates vicinal electrophilic sites readily available for a broad range of nucleophiles.
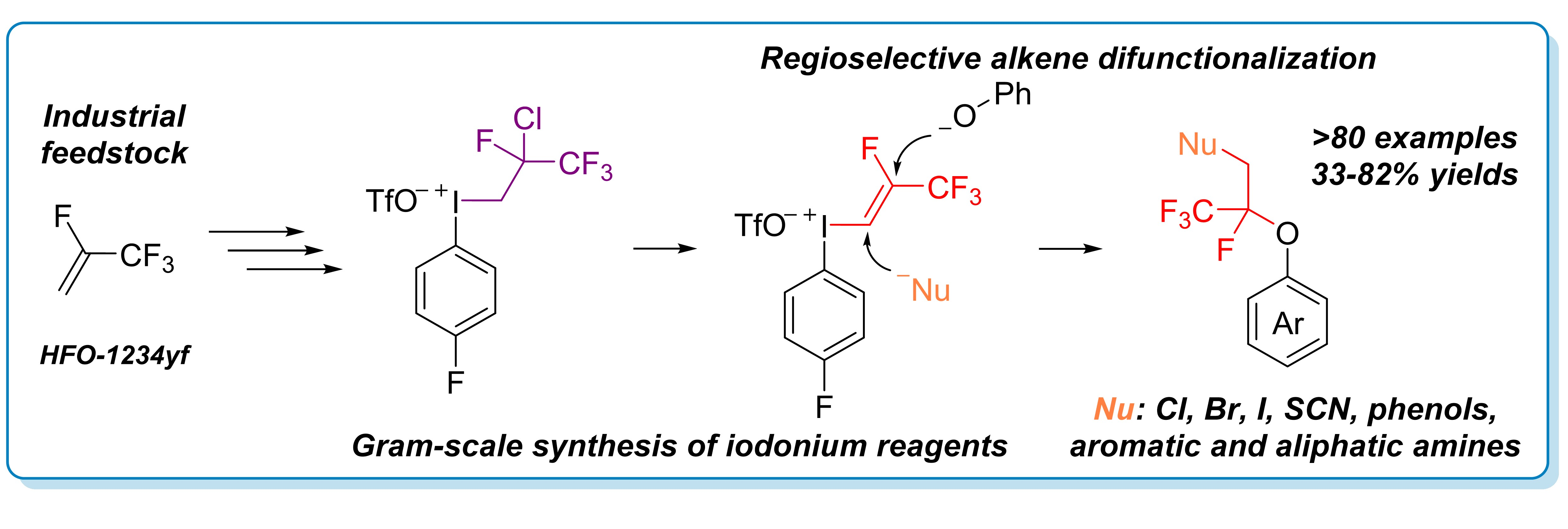
93. Photoinduced Decarboxylative Borylation of N‑Hydroxyphthalimide Esters with Hypoboric Acid
Photoinduced Decarboxylative Borylation of N‑Hydroxyphthalimide Esters with Hypoboric Acid, Bálint Nagy, Zsombor Gonda, Tamás Földesi, Péter Pál Fehér, András Stirling, Gergely L. Tolnai, Zoltán Novák, Org. Lett. 2024, 26, 2292–2296. DOI: 10.1021/acs.orglett.4c00511 | [Full Text Link] [Supp. Info Link]
We developed a visible-light driven photochemical transformation, in which activated primary, secondary and tertiary alkylcarboxylic acids were converted into the corresponding boronic esters in the absence of catechol and any added photocatalyst. The procedure relies on the utilization of hypoboric acid and redox-active esters of alkylcarboxylic acids, ensuring simple and economic procedure. Quantum chemical calculations and mechanistic considerations provide deeper insights into the mechanism of photochemical borylation reactions.