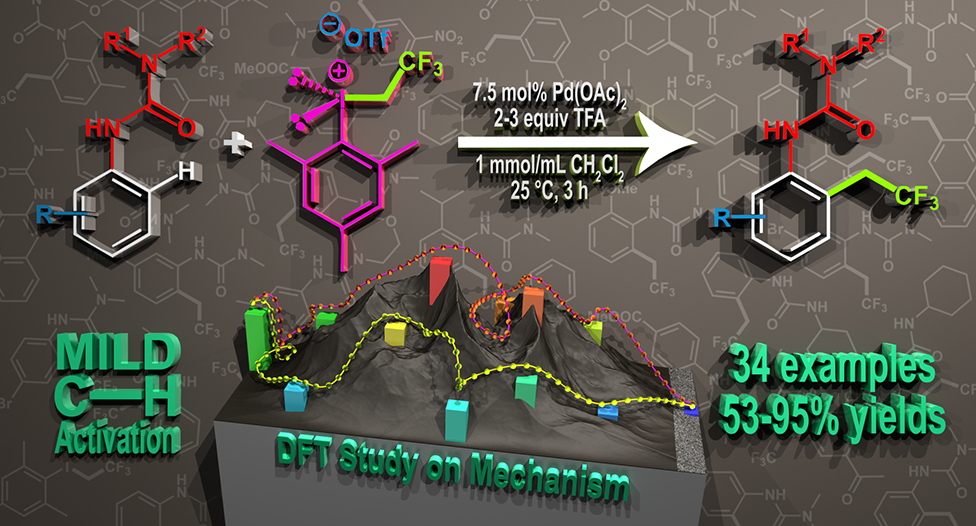
57. Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions
Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions, Szabolcs Kovács, Balázs L. Tóth, Gábor Borsik, Tamás Bihari, Nóra V. May, András Stirling, Zoltán Novák, Adv. Synth. Catal. 2017, 359, 527-532. DOI: 10.1002/adsc.201601136 | [Full Text Link] [Supp. Info. Link]
Abstract: Development of direct late-stage installation of alkyl groups into aromatic systems is an important and challenging task of current organic chemistry. In spite of the existing functionalization methods in organic chemistry for the substitution reactions on aromatic systems, the direct alkylation of aromatic ureas is unknown. Herein, as a first example we report a novel palladium catalyzed fluoroalkylation process by C−H activation for the access of ortho trifluoroethylated aromatic ureas. The application of novel, highly active trifluoroethyl(mesityl)iodonium salt enables the efficient introduction of the trifluoroethyl group at 25 °C in 3 hours in high yields (up to 95%) with good functional group tolerance. DFT calculations have revealed a rate determining oxidative alkyl-group transfer preceded by an unexpected C−H activation route on the Pd center during the catalytic cycle, where the deprotonation is assisted by an external triflate anion.
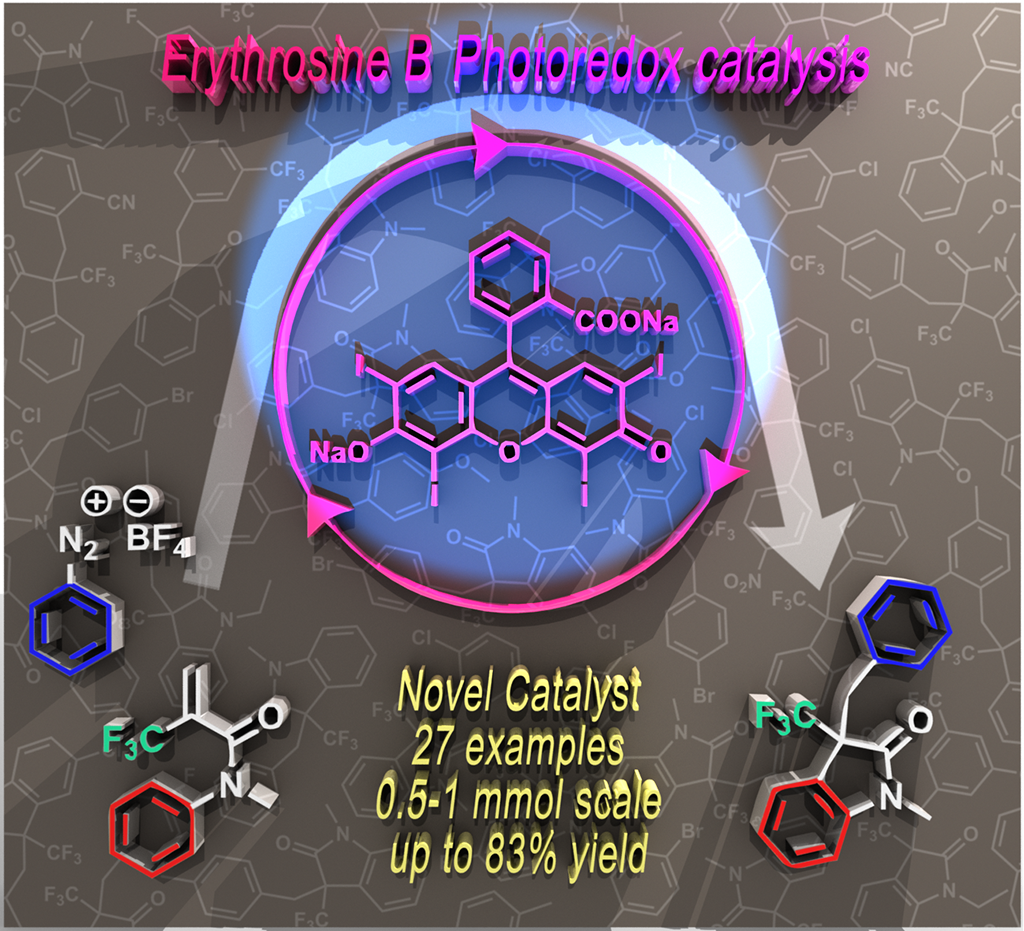
58. Erythrosine B catalyzed visible-light photoredox arylation-cyclization of N-alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(trifluoromethyl)indolin-2-one derivatives
Erythrosine B catalyzed visible-light photoredox arylation-cyclization of N-alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(trifluoromethyl)indolin-2-one derivatives, Zsombor Gonda, Ferenc Béke, Orsolya Tischler, Milán Petró, Zoltán Novák, Balázs L. Tóth, Eur. J. Org. Chem. 2017, 15, 2112-2117. DOI: 10.1002/ejoc.201601493 | [Full Text Link] [Supp. Info. Link]
Special Issue: Photoredox Catalysis
3-Trifluoromethyl-indoline-2-one derivatives were prepared in a visible-light photocatalytic transformation of acrylamides. The arylation-ring closure was initiated by light induced aryl radical generation from aryl diazonium salts with the utilization of erythrosine B as novel organic photocatalyst.
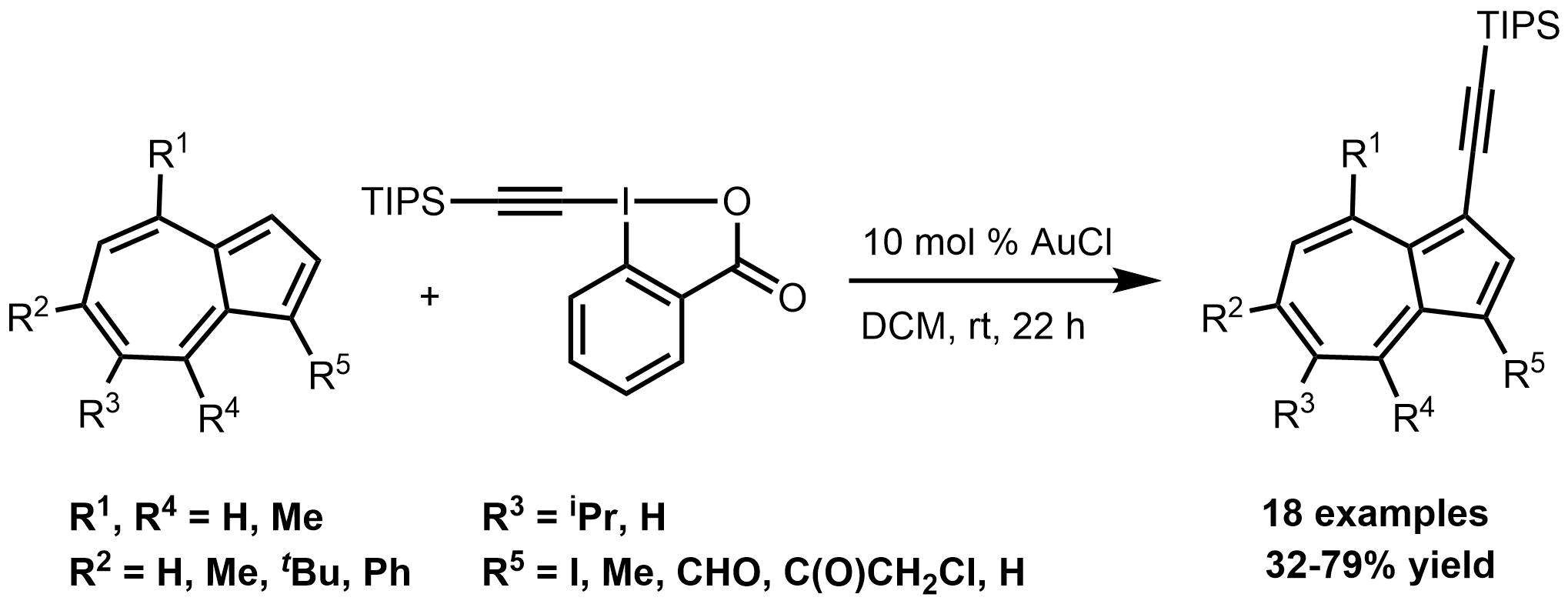
59. Gold-Catalyzed Direct Alkynylation of Azulenes
Gold-Catalyzed Direct Alkynylation of Azulenes, Anna Székely, Áron Péter, Klára Aradi, Gergely L. Tolnai, Zoltán Novák, Org. Lett. 2017, 19, 954-957. DOI: 10.1021/acs.orglett.7b00259 | [Full Text Link] [Supp. Info. Link]
A novel catalytic method for the direct C-H alkynylation of azulenes was developed. The gold catalyzed functionalization of this special carbacycle iwas achieved with hypervalent iodonium reagent TIPS-EBX under mild reaction conditions. With the aid of the developed procedure, several TIPS alkynylated azulene derivatives were synthesized bearing important func-tional groups for further functionalization.
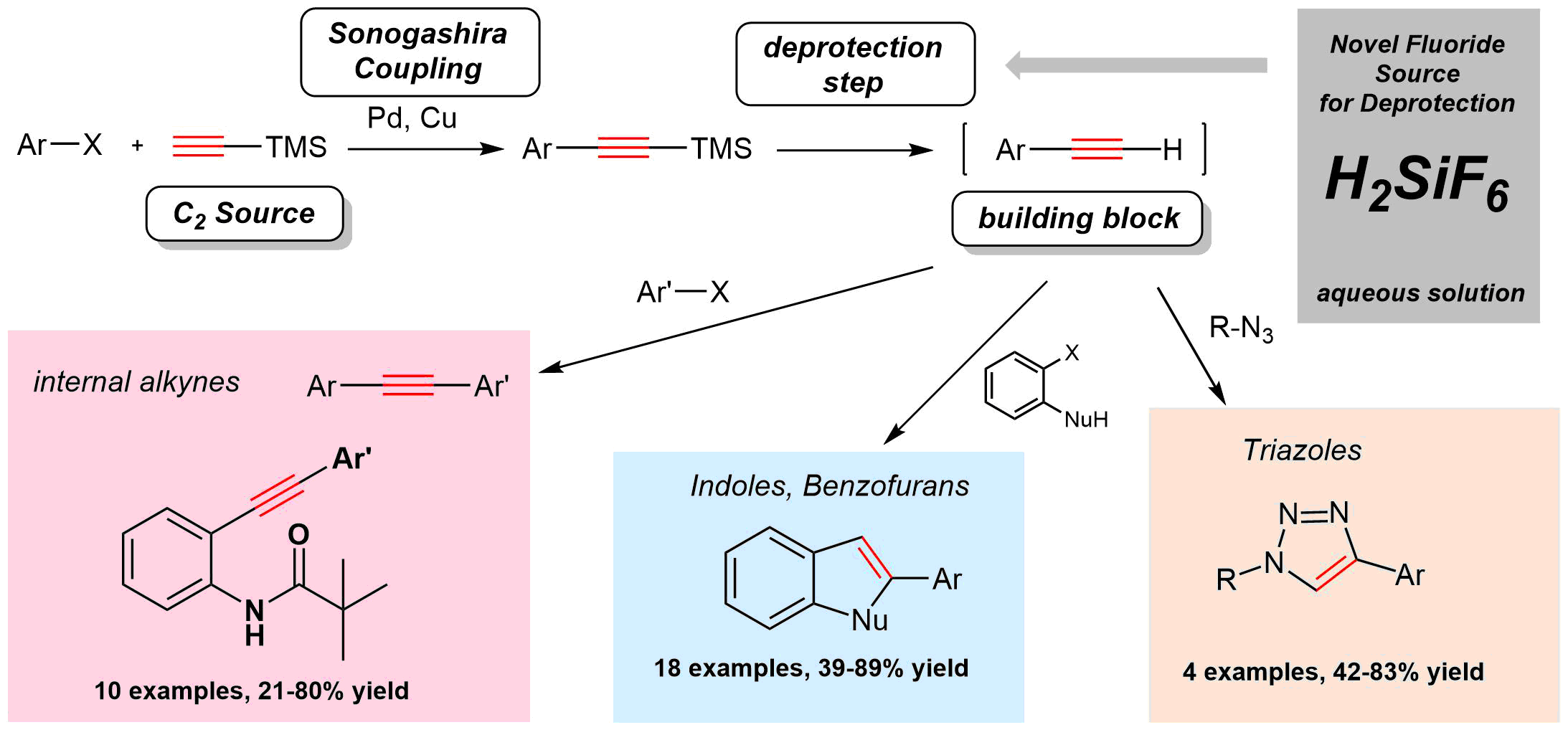
60. Hexafluorosilicic Acid as a Novel Reagent for the Desilylation of Silylacetylenes: Application in Sequential Sonogashira Coupling and Click Reaction
Hexafluorosilicic Acid as a Novel Reagent for the Desilylation of Silylacetylenes: Application in Sequential Sonogashira Coupling and Click Reaction, Ádám Sinai, Ádám Mészáros, Ádám Balogh, Márton Zwillinger, Zoltán Novák, Synthesis 2017, 49, 2374-2388. DOI: 10.1055/s-0036-1588981 | [Full Text Link] [Supp. Info. Link]
Key words: alkynes - azides - cycloaddition - click reaction - cross-coupling - fluoride - palladium - heterocycles
Hexafluorosilicic acid was utilized as a novel, cheap, readily available, and environmentally benign alternative reagent for the desilylation of 1-trimethylsilylacetylenes. The applicability of the aqueous solution of the hexafluorosilicic acid was demonstrated in the sequential coupling of aryl halides and ethynyltrimethylsilane to afford internal acetylenes, benzofurans, and triazoles in one-pot Sonogashira–Sonogashira and Sonogashira–CuAAC reactions.
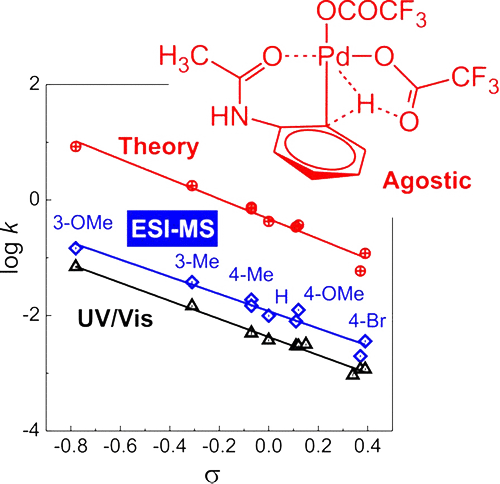
61. Palladium-Catalyzed C–H Activation: Mass Spectrometric Approach to Reaction Kinetics in Solution
Palladium-Catalyzed C–H Activation: Mass Spectrometric Approach to Reaction Kinetics in Solution, Jiří Váňa, Thibault Terencio, Vladimir Petrović, Orsolya Tischler, Zoltán Novák, Jana Roithová, Organometallics 2017, 36, 2072-2080. DOI: 10.1021/acs.organomet.6b00960 | [Full Text Link] [Supp. Info. Link, XYZ]
We report a new method for determination of rate constants of processes in solution using electrospray ionization mass spectrometry (ESI-MS). The investigated reaction is C–H activation of acetanilides by palladium(II)trifluoroacetate leading to stable organopalladium complexes. The rate constants can be determined from an experiment with a couple of differently substituted acetanilides being in competition for being activated by the palladium salt. The formed organopalladium complexes can be detected by ESI-MS. The time dependence is achieved by adding one of the acetanilides to the reaction mixture with a time delay. The kinetics can be then evaluated from the evolution of the ratio of the ESI-MS signals of differently substituted complexes as a function of the time delay. The Hammett analysis of the rate constants obtained for a series of meta- and para-substituted acetanilides provides a ρ value of −1.5, which is in agreement with values reported for similar C–H activations. We have also investigated the very same reaction with UV–vis spectroscopy that gave us about three times smaller rate constants but the same trend with the ρ value of −1.6. The rate constants determined by ESI-MS are directly linked to the occurrence of organopalladium complexes, whereas the UV–vis data are associated with an absorption spectra change that could involve more reaction steps. DFT calculations support the interpretation of the reaction mechanism as cyclopalladation and provide the ρ value in the same range. The rate-determining step corresponds to the agostic C–H transition structure.