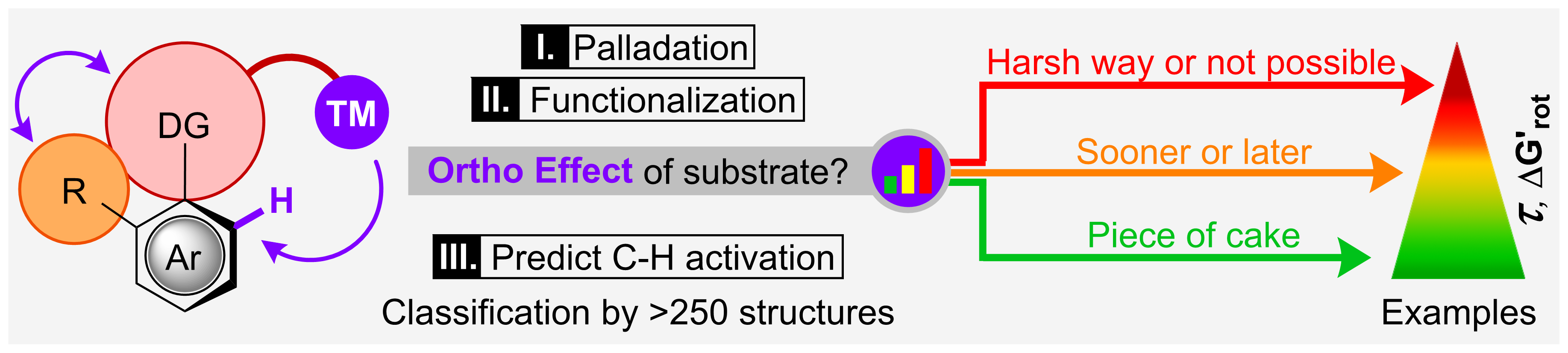
79. The Ortho Effect in Directed C-H Activation
The Ortho Effect in Directed C–H Activation, Balázs L. Tóth, Anna Monory, Orsolya Egyed, Attila Domján, Attila Bényei, Bálint Szathury, Zoltán Novák, András Stirling, Chem. Sci. 2021, 12, 5152-5163. Open Access. DOI: 10.1039/D1SC00642H | [Full Text Link] [Supp. Info. Link] [Full Interactive Database (XLSX)] [Crystal Data (CIF)] [Structures 1 (XYZ)] [Structures 2 (XYZ)]
The success of transition metal-catalysed ortho-directed C–H activation is often plagued by the effects of undesirable interactions between the directing group (DG) and other groups introduced into the aromatic core of the substrate. In particular, when these groups are in neighbouring positions, their interactions can affect profoundly the efficacy of the C–H activation by transition metals. In this work we introduce a simple substrate-only-based model to interpret the influence of steric hindrance of a group in ortho position to the DG in directed ortho-C–H bond activation reactions, and coined the term Ortho Effect (OE) for such situations. We consider simple descriptors such as torsion angle and torsional energy to predict and explain the reactivity of a given substrate in directed C–H activation reactions. More than 250 examples have been invoked for the model, and the nature of the ortho effect was demonstrated on a wide variety of structures. In order to guide organic chemists, we set structural and energetic criteria to evaluate a priori the efficiency of the metalation step which is usually the rate-determining event in C–H activations, i.e. we provide a simple and general protocol to estimate the reactivity of a potential substrate in C–H activation. For borderline cases these criteria help set the minimum reaction temperature to obtain reasonable reaction rates. As an example for the practical applicability of the model, we performed synthetic validations via palladium-catalysed 2,2,2-trifluoroethylation reactions in our lab. Furthermore, we give predictions for the necessary reaction conditions for several selected DGs.
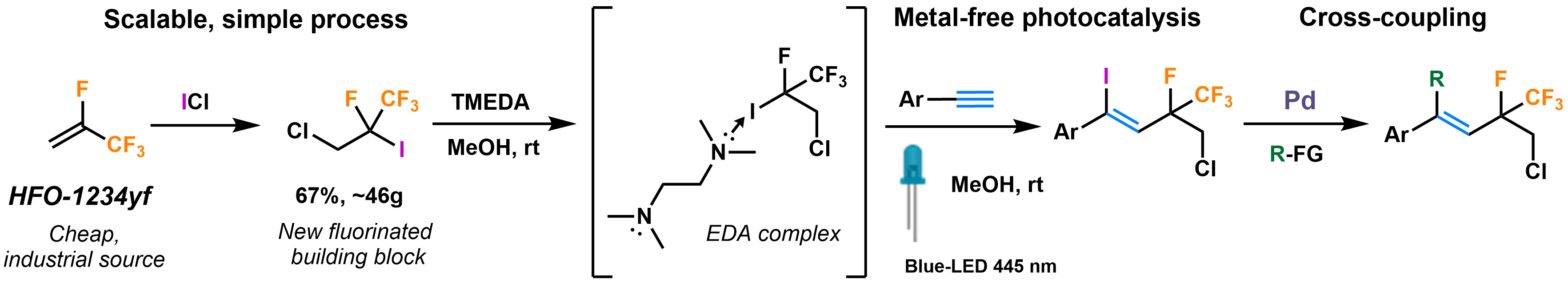
80. Synthesis and Photochemical Application of Hydrofluoroolefin (HFO) Based Fluoroalkyl Building Block
Synthesis and Photochemical Application of Hydrofluoroolefin (HFO) Based Fluoroalkyl Building Block, Bálint Varga, Balázs L. Tóth, Ferenc Béke, János T. Csenki, András Kotschy, Zoltán Novák, Org. Lett. 2021, 23, 4925–4929. DOI: 10.1021/acs.orglett.1c01709 | [Full Text Link] [Supp. Info. Link]
A novel fluoroalkyl iodide was synthesized on multigram scale from refrigerant gas HFO-1234yf as cheap industrial starting material in a simple, solvent-free, and easily scalable process. We demonstrated its applicability in a metal-free photocatalytic ATRA reaction to synthesize valuable fluoroalkylated vinyl iodides and proved the straightforward transformability of the products in cross-coupling chemistry to obtain conjugated systems.
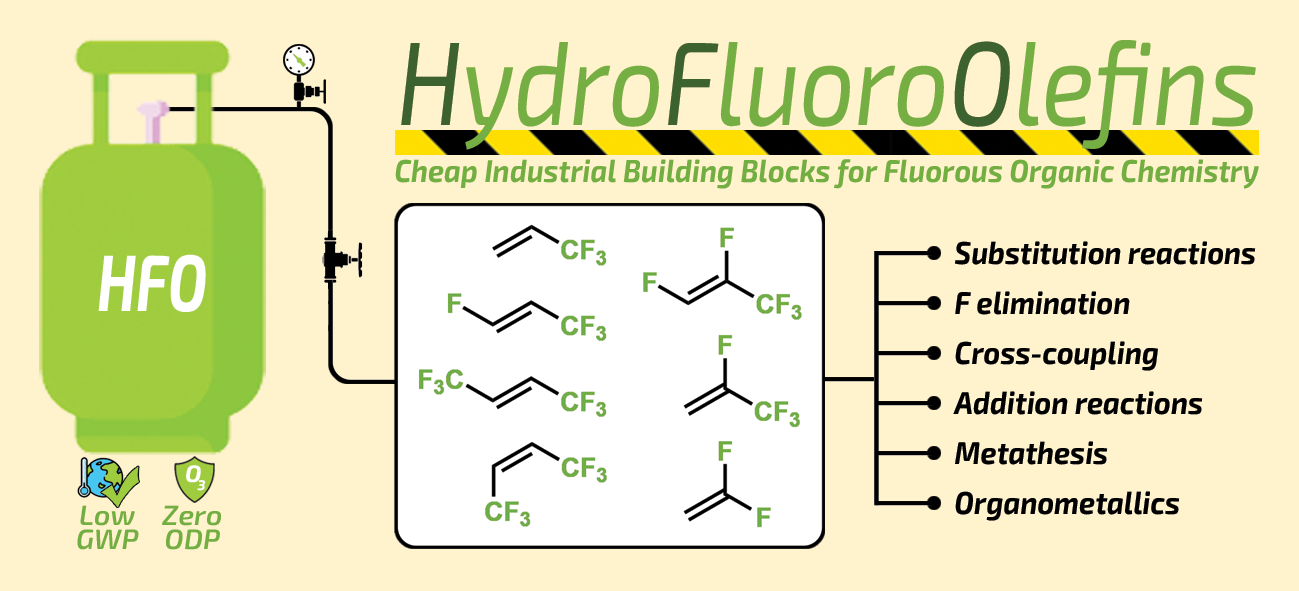
81. Application of industrially relevant HFO gases in organic syntheses
Application of industrially relevant HFO gases in organic syntheses, Bálint Varga, János Csenki, Balázs L. Tóth, Ferenc Béke, Zoltán Novák, András Kotschy, Synthesis 2021, 53, 4313-4326. DOI: 10.1055/a-1538-8344 | [Full Text Link]
Hydrofluoroolefin (HFO) gases are state-of-the-art cooling agents with widespread household and industrial applications. Considering their structural benefits these fluorous feedstocks gained attention of organic chemists in the last couple of years. In this short review we summarized the existing synthetic transformations of these gaseous starting material and present their applicability in the synthesis of fluorine containing organic molecules, which have potential importance as building blocks and reagents for diverse syntheses.
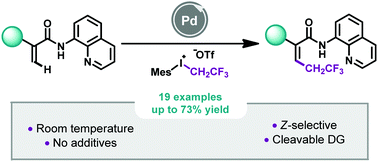
82. Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature
Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature, Louise Ruyet, Maria I. Lapuh, Vijay S. Koshti, Tamás Földesi, Philippe Jubault, Thomas Poisson, Zoltán Novák, Tatiana Besset, Chem. Commun. 2021, 57, 6241-6244. DOI: 10.1039/D1CC02007B | [Supp. Info. Link]
A straightforward 2,2,2-trifluoroethylation of acrylamides by Pd-catalyzed C–H bond activation was reported by using a fluorinated hypervalent iodine reagent as a coupling partner. At room temperature, this additive-free approach allowed the synthesis of Z-2,2,2-trifluoroethylated acrylamides (19 examples, up to 73% yield) in a stereoselective manner. Under these mild reaction conditions, the methodology turned out to be functional group tolerant and mechanistic studies gave us a better understanding of the transformation.
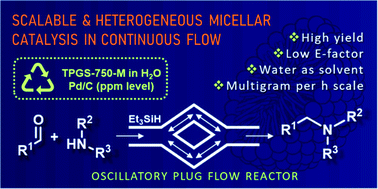
83. Continuous flow heterogeneous catalytic reductive aminations under aqueous micellar conditions enabled by an oscillatory plug flow reactor
Continuous flow heterogeneous catalytic reductive aminations under aqueous micellar conditions enabled by an oscillatory plug flow reactor, Michaela Wernik, Gellért Sipos, Balázs Buchholcz, Ferenc Darvas, Zoltán Novák, Sándor B. Ötvös, C. Oliver Kappe, Green Chem. 2021, 23, 5625-5632. DOI: 10.1039/D1GC02039K | [Full Text Link] [Supp. Info. Link]
Despite the fact that continuous flow processing exhibits well-established technical advances, aqueous micellar chemistry, a field that has proven extremely useful in shifting organic synthesis to sustainable water-based media, has mostly been explored under conventional batch-based conditions. This is particularly because of the fact that the reliable handling of slurries and suspensions in flow has been considered as a significant technical challenge. Herein, we demonstrate that the strategic application of an oscillatory plug flow reactor enables heterogeneous catalytic reductive aminations in aqueous micellar media enhancing mass transport and facilitating process simplicity, stability and scalability. The micellar flow process enabled a broad range of substrates, including amino acid derivatives, to be successfully transformed under reasonably mild conditions utilizing only very low amounts of Pd/C as a readily available heterogeneous catalyst. The preparative capabilities of the process along with the recyclability of the heterogenous catalyst and the aqueous reaction media were also demonstrated.