86. Z-Selective Fluoroalkenylation of (Hetero)Aromatic Systems by Iodonium Reagents in Palladium-Catalyzed Directed C-H Activation
Z-Selective Fluoroalkenylation of (Hetero)Aromatic Systems by Iodonium Reagents in Palladium-Catalyzed Directed C-H Activation, Balázs L. Tóth, Gergő Sályi, Attila Domján, Orsolya Egyed, Attila Bényei, Zsombor Gonda, Zoltán Novák, Adv. Synth. Catal. 2022, 364, 348-354. DOI: 10.1002/adsc.202101108 | [Full Text Link] [Supp. Info. Link]
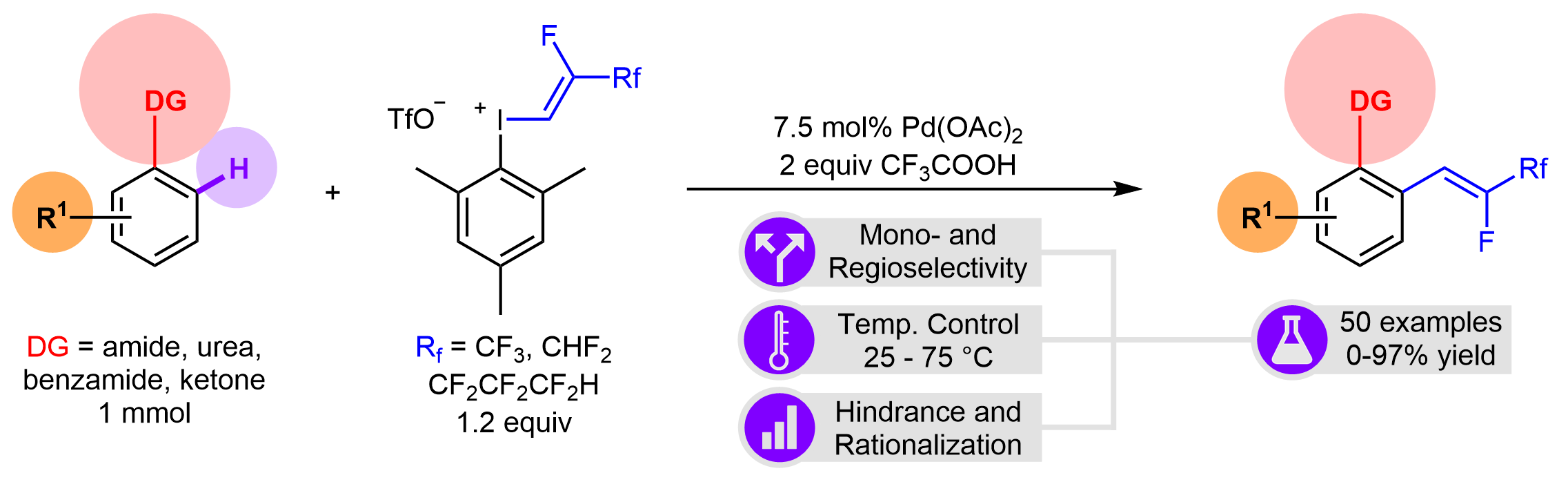
The direct and catalytic incorporation of fluorine containing molecular motifs into organic compounds resulting high-value added chemicals represents a rapidly evolving part of synthetic methodologies, thus this area is in the focus of pharmaceutical and agrochemical research. Herein we report a stereoselective procedure for direct fluorovinylation of aromatic and heteroaromatic scaffolds. This methodology development has been realized by palladium-catalyzed ortho C-H activation reaction of aniline derivatives featuring the regioselectivity via directing groups such as secondary of tertiary amides, ureas or ketones. The application of non-symmetrical aryl(fluoroalkenyl)-iodonium salts as fluoroalkenylating agents allowed mild reaction conditions in general for this transformation. The scope and limitations have been thoroughly investigated and the feasibility has been demonstrated by more than 50 examples.
87. The Potential of Micellar Media in the Synthesis of DNA-Encoded Libraries
The Potential of Micellar Media in the Synthesis of DNA-Encoded Libraries, Réka Adamik, Balázs Buchholcz, Ferenc Darvas, Gellért Sipos, Zoltán Novák, Chem. Eur. J. 2022, 28, e202103967. DOI: 10.1002/chem.202103967 | [Full Text Link]
DNA-encoded libraries (DELs) have become a widely used technology in drug discovery. The use of micelle-forming surfactants provides new possibilities for synthetic organic chemistry and could be a promising technique for DNA-compatible chemistry as well. This review provides a summary of existing examples of micellar DEL approaches, together with a selection of micellar organic transformations fundamentally suitable for DEL synthesis.
Highlighted as a Frontispiece. Chem. Eur. J. 2022, 28, e202282062. DOI: 10.1002/chem.202282062 | [Full Text Link]
88. Synthesis of Hydrofluoroolefin-Based Iodonium Reagent via Dyotropic Rearrangement and Its Utilization in Fluoroalkylation
Synthesis of Hydrofluoroolefin-Based Iodonium Reagent via Dyotropic Rearrangement and Its Utilization in Fluoroalkylation, János T. Csenki, Balázs L. Tóth, Ferenc Béke, Bálint Varga, Péter P. Fehér, András Stirling, Zsuzsanna Czégény, Attila Bényei, Zoltán Novák, Angew. Chem. Int. Ed. 2022, 61, ASAP. DOI: 10.1002/anie.202208420 | [Full Text Link] [Supp. Info Link] [Crystal Data (CIF)]
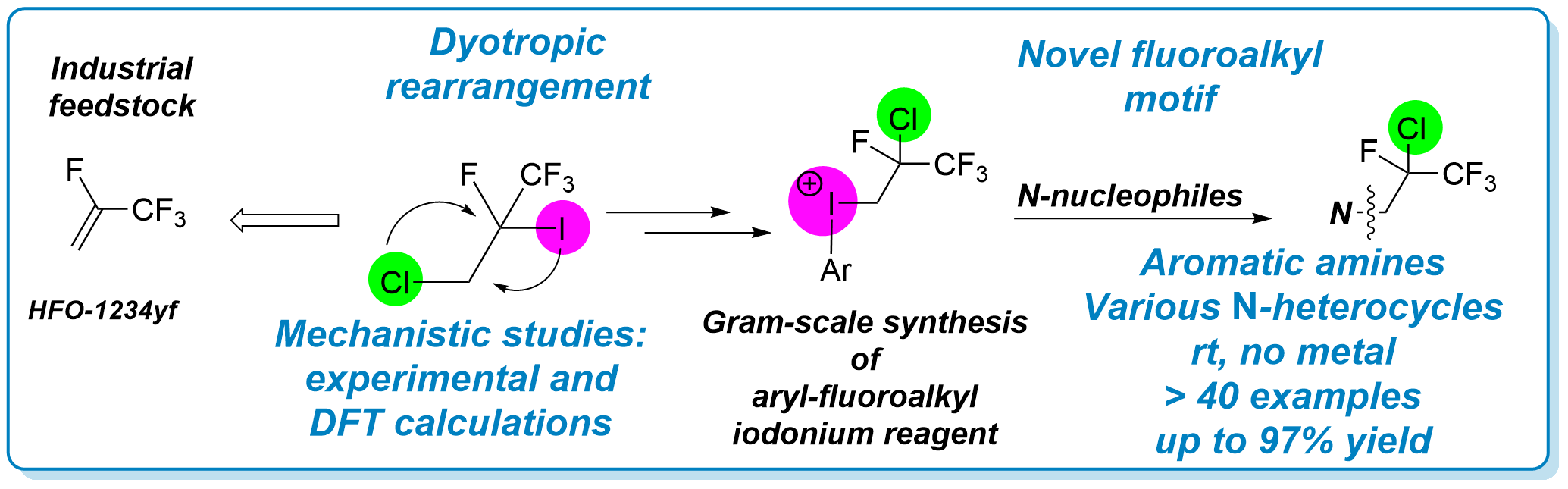
The synthesis of a novel hypervalent iodonium salt was developed with the exploitation of a dyotropic rearrangement of iodine and chlorine functions in fluoroalkyl chains from HFO-1234yf gas. The electrophilic reagent enabled the direct and metal-free introduction of a special fluoroalkyl group into aniline derivatives and N-containing heteroaromatic compounds. Detailed experimental and computational data were collected and used to propose a mechanistic description of this unique rearrangement.
89. Steric Effects in ortho C-H Activation of Aromatic Systems
Steric Effects in ortho C-H Activation of Aromatic Systems, Z. Novák, B. L. Tóth, A. Stirling, Handbook of C-H functionalization. Chapter: Role of Directing Groups. 2022, Wiley. DOI: 10.1002/9783527834242.chf0080 | [Full Text Link] [Table of Content of The Book]
Transition metal-catalyzed directed aromatic ortho C-H activation offers many possibilities for the functionalization of various substrates having different directing groups. However, the reactivity of the substrate is determined by electronic and steric factors. Herein, we present and discuss a recently developed model which considers only the steric properties of the substrates to assess their reactivity in ortho C-H activation in the presence of ortho substituents. Dihedral angle between the directing group and the aromatic ring influences the coordination and the C-H activation elementary step, thus determine reactivities and the stability of metal-complex intermediates. The presentation of the theoretical model is followed by several literature examples from substrates having borderline reactivities, to explore and describe the most interesting region of reactivity. The most commonly used directing groups are discussed taking into consideration their key structural elements, and reactivity trends are presented with the help of complex formation and reactions of each substrate group. The lack of transformation is also discussed by showing representatives of unreactive substrates.

