54. Continuous flow synthesis of heterocyclic scaffolds Design principles of multistep systems – A review
Continuous flow synthesis of heterocyclic scaffolds. Design principles of multistep systems – A review, Klára Lövei, Péter Bana, Róbert Örkényi, György I. Túrós, János Éles, Zoltán Novák, Ferenc Faigl, Chim. Oggi Chem. Today, 2016, 34, 18-21. Link
KEYWORDS: Continuous flow synthesis, condensed heterocycles, scaffold, multistep flow synthesis, telescoping.
ABSTRACT: The synthesis of novel heterocycles is an essential task in small-molecule drug discovery. Continuous flow processing opens the way for a new paradigm in laboratory-scale synthesis as well as pharmaceutical manufacturing. Based on our experiences with the multistep synthesis of condensed benzothiazoles, we gathered some of the key design features in light of literature examples.
51. Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies
Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies, Klára Aradi, Balázs L. Tóth, Gergely L. Tolnai, Zoltán Novák, Synlett 2016, 27, 1456-1485. DOI: 10.1055/s-0035-1561369 | [Full Text Link]
Keywords: arylation - diaryliodonium salts - hypervalent iodine reagents - metal-free - copper-catalyzed - palladium-catalyzed - C–H activation - direct functionalization
This account aims to give a description of the usefulness of diaryliodonium salts in organic chemistry, including their synthesis and applications in the presence and absence of transition-metal catalysts. Herein, we briefly summarize the structural properties and reactivity of diaryliodonium salts. We describe several applications of these hypervalent reagents including metal-free arylations of C-, O-, N- and S-nucleophiles. The synthesis and functionalization of aromatic and heteroaromatic systems via copper- and palladium-catalyzed transformations are also discussed in this account.
Content:
1 Introduction
2 Structural Properties and Reactivity
3 Applications of Diaryliodonium Salts in Organic Syntheses
3.1 Transition-Metal-Free Arylations of Heteroatom and Carbon Nucleophiles
3.1.1 Arylation of Oxygen Nucleophiles
3.1.1.1 Arylation of (Hetero)aromatic Alcohols
3.1.1.2 Arylation of Aliphatic Alcohols
3.1.1.3 Arylation of Acids
3.1.2 Arylation of Nitrogen Nucleophiles
3.1.2.1 N-Arylation of Amines and Amides
3.1.2.2 N-Arylation of Heterocycles
3.1.3 Arylation of Sulfur Nucleophiles
3.1.4 Arylation of Inorganic Anions
3.1.5 Arylation of Carbon Nucleophiles
3.1.5.1 Electron-Rich Heterocycles
3.1.5.2 Arylation of Carbonyl and Nitro Compounds
3.2 Synthesis and Functionalization of Aromatic and Heteroaromatic Molecules with Diaryliodonium Salts in the Presence of Copper and Palladium Catalysts
3.2.1 C–H Functionalizations by Copper-Catalyzed C–H Arylations
3.2.2 Copper-Catalyzed Cyclization of Unsaturated Compounds with Diaryliodonium Salts
3.2.3 Palladium-Catalyzed Arylations
3.2.3.1 Electrophilic Palladation and Arylation
3.2.3.2 Directing-Group-Assisted C–H Bond Activation and Arylation
4 Conclusion
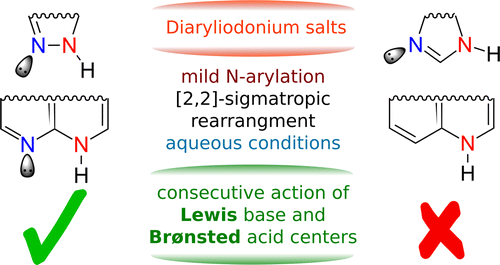
52. Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N‑Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study
Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N‑Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study, Tamás Bihari, Bence Babinszki, Zsombor Gonda, Szabolcs Kovács, Zoltán Novák, András Stirling, J. Org. Chem. 2016, 81, 5417-5422. DOI: 10.1021/acs.joc.6b00779 | [Full Text Link] [Supp. Info. Link]
The mechanism of arylation of N-heterocycles with unsymmetric diaryliodonium salts is elucidated. The fast and efficient N-arylation reaction is interpreted in terms of the bifunctionality of the substrate: The consecutive actions of properly oriented Lewis base and Brønsted acid centers in sufficient proximity result in the fast and efficient N-arylation. The mechanistic picture points to a promising synthetic strategy where suitably positioned nucleophilic and acidic centers enable functionalization, and it is tested experimentally.
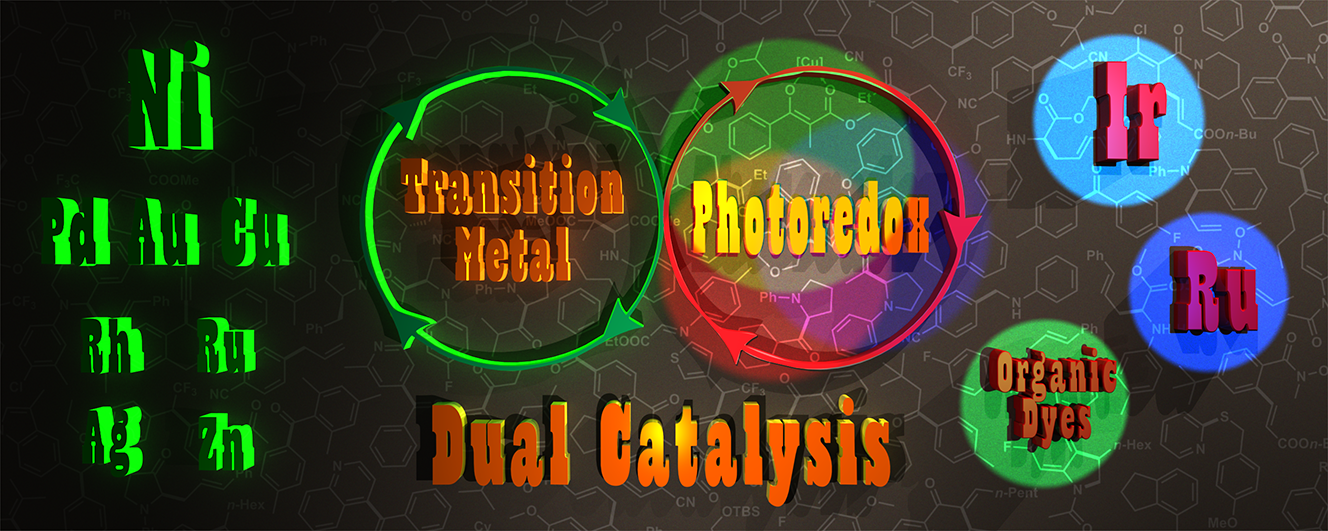
53. Recent advances in dual transition metal–visible light photoredox catalysis
Recent advances in dual transition metal–visible light photoredox catalysis, Balázs L. Tóth, Orsolya Tischler, Zoltán Novák, Tetrahedron Lett. 2016, 57, 4505–4513. DOI: 10.1016/j.tetlet.2016.08.081 |
In this Digest Letter, we collected and shortly summarized the recently developed dual transition metal–visible light photoredox catalytic processes including arylation, alkynylation, alkenylation, allylation, alkylation, fluoroalkylation, benzylation, acylation, and cyclization reactions. The utilization of multimetallic catalytic systems provides new synthetic strategies for the synthesis and functionalization of versatile and novel organic compounds.
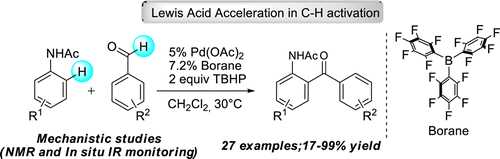
50. Activation of C–H Activation: The Beneficial Effect of Catalytic Amount of Triaryl Boranes on Palladium-Catalyzed C–H Activation
Activation of C–H Activation: The Beneficial Effect of Catalytic Amount of Triaryl Boranes on Palladium-Catalyzed C–H Activation, Orsolya Tischler, Zsófia Bokányi, Zoltán Novák, Organometallics 2016, 35, 741-746. DOI: 10.1021/acs.organomet.5b01017 | [Full Text Link] [Supp. Info. Link]
Herein we report a novel approach to the acceleration of palladium-catalyzed C–H activation reactions. We demonstrated that the utilization of electron-deficient triaryl boranes as Lewis acidic cocatalysts of palladium enables the directed cross dehydrogenative coupling of aldehydes and anilides under mild reaction conditions. Study of the kinetic profile of the transformation reveals a unique, unexpectedly long induction period of the transformation.