91. Fluoroalkylations and Fluoroalkenylations with Iodonium Salts
Fluoroalkylations and Fluoroalkenylations with Iodonium Salts, Ferenc Béke, János T. Csenki, Zoltán Novák, Chem. Rec. 2023, 23, e202300083. DOI: 10.1002/tcr.202300083 | [Full Text Link]
Synthesis and applications of fluoroalkyl and fluoroalkenyliodonium salts are summarized in this account article, focusing preferably to the reagents designed in our laboratory in the last decade. Among these reagents trifluoroethyl(aryl)iodonium salts have been used most frequently to build carbon-carbon and carbon-heteroatom bonds in simple nucleophilic substitutions and through transition metal catalyzed coupling reactions. Iodonium salts equipped with unsaturated fluorinated function showed diverse reactivity due to their electron deficient character, and these molecular motifs enable cycloadditions and nucleophilic additions to prepare fluorinated carbo- and heterocyclic molecules. Beyond the overview of existing transformations, with the presented collection, we aim to inspire future developments of iodonium reagents and their application in organic synthesis.
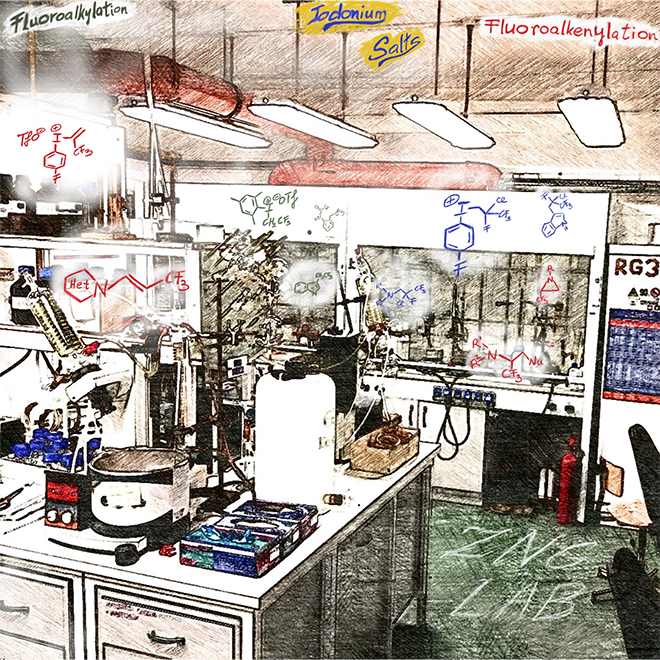
90. Gate to Parallel Universe: Utilization of Biosurfactants in Micellar Catalysis
Gate to Parallel Universe: Utilization of Biosurfactants in Micellar Catalysis, Réka Adamik, Attila R. Herczegh, Imre Varga, Zoltán May, Zoltán Novák, Green Chem.. 2023, ASAP. DOI: 10.1039/D3GC00365E | [Full Text Link] [Supp. Info Link]
Micellar catalysis offers a green alternative media for catalytic transformations by solubilization of hydrophobic organic compounds. Biosurfactants represent a sustainable alternative to synthetic surfactants due to their biodegradability, low toxicity, and production from renewable feedstocks. In this work, we demonstrated the applicability of biosurfactant rhamnolipids in various micellar cross couplings, and prepared special fluorinated conjugated systems, Active Pharmaceutical Ingredient (API) building blocks and bioactive compounds