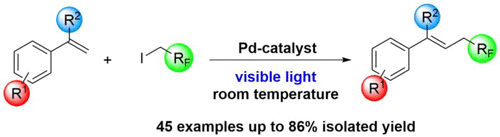
Turn Industrially Relevant Gasous HFO-1234yf To Iodonium Salts
Besides the application of hypervalent iodine reagents, a new type of dyotropic rearrangement has been discovered. The work has been published in Angewandte Chemistry International Edition (Wiley). For further reading, please, click here.
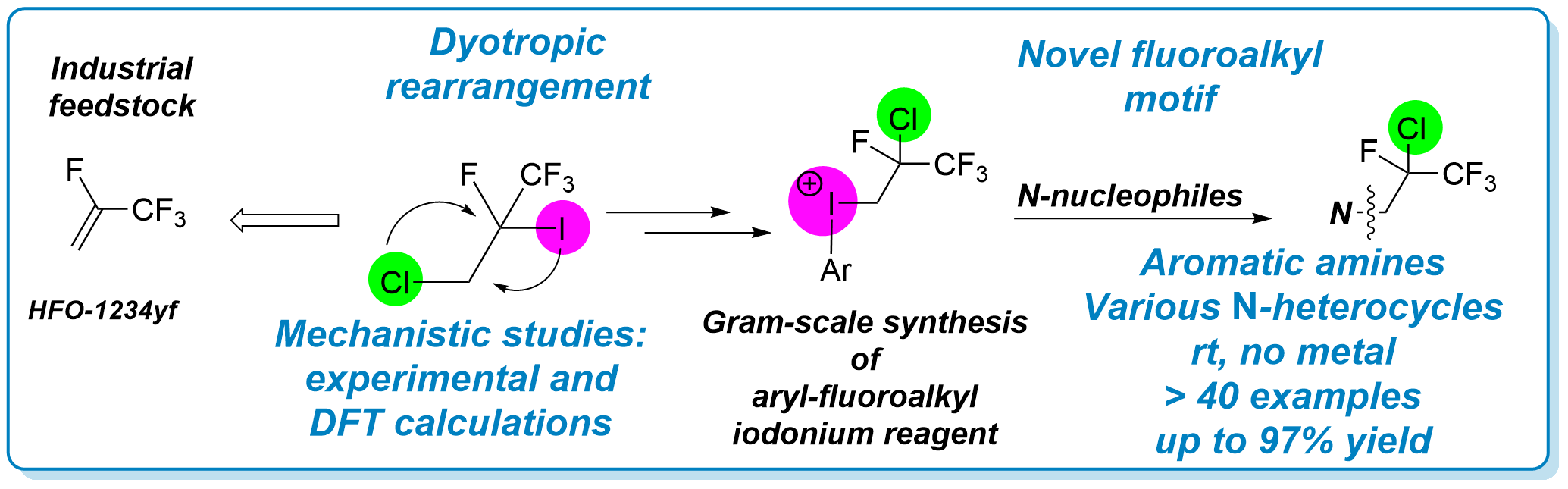
Curse or blessing? Influence of impurities on cross-coupling
Our new practical guideline for elucidating catalysts has been published in Nature Catalysis as a matters arising article. For further reading, please, click here.
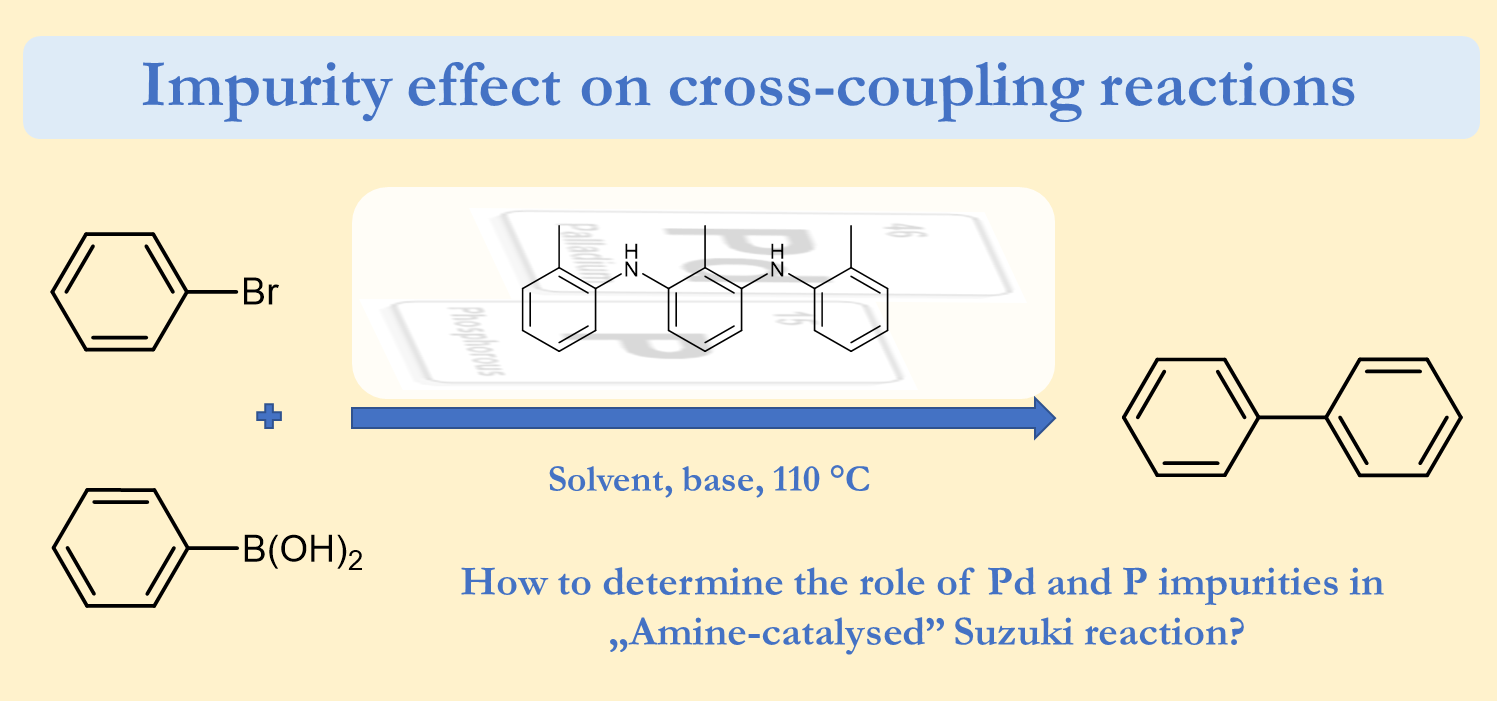
Revealing the Mysterious Effect of Ortho Substituents in Aromatic C-H Activation
Our new theoretical and practical study has been published in Chemical Science (RSC) as an Edge Article. In this investigation we aimed to explain in details the Ortho Effect in Transitional Metal Catalyzed C-H Activation. To provide a comprehensive overview. More than 250 substrates were added to the downloadable Fully Interactive Database. The developed methodology was validated in conceptual experiments, allowing reliable prediction for C-H Activation of custom substrates. For further reading, please, click here.
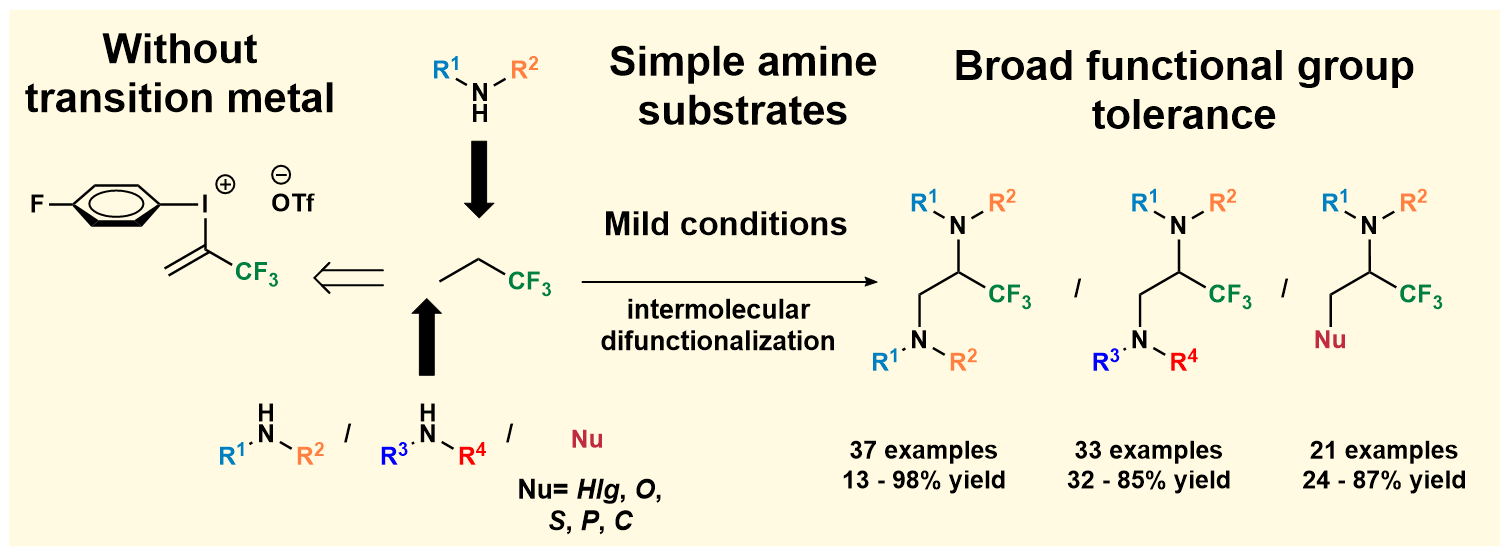
Methodology Development for Preparation of Pharmaceutically Relevant Trifluoroalkyl Diamines
We have published our new work about metal-free vicinal difunctionalization of carbon-carbon double bond for the platform synthesis of trifluoroalkyl amines in Nature Communications (Nature Research). For further reading, please, click here.
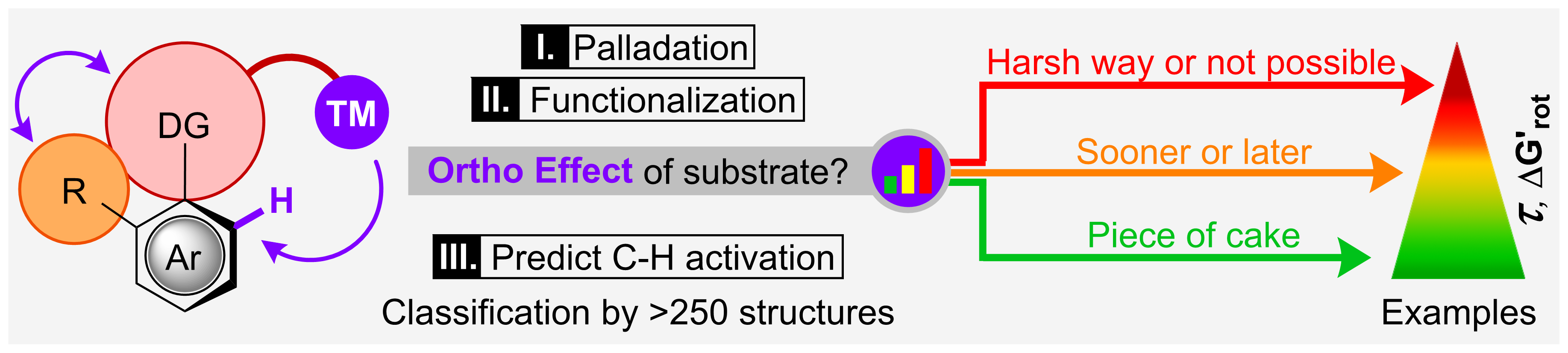
Fluoroalkylation Under Mild Photoredox Conditions
Our paper about Photocatalytic Palladium-Catalyzed Fluoroalkylation of Styrene Derivatives has been published in Organic Letters (ACS). For further reading, please, click here.