64. Sulfonium Salts as Alkylating Agents for Palladium-Catalyzed Direct Ortho Alkylation of Anilides and Aromatic Ureas
Sulfonium Salts as Alkylating Agents for Palladium-Catalyzed Direct Ortho Alkylation of Anilides and Aromatic Ureas, Dániel Cs. Simkó, Péter Elekes, Vivien Pázmándi, Zoltán Novák, Org. Lett. 2018, 20, 676-679. DOI: 10.1021/acs.orglett.7b03813 | [Full Text Link] [Supp. Info. Link]
A novel method for the ortho alkylation of acetanilide and aromatic urea derivatives via C–H activation was developed. Alkyl dibenzothiophenium salts are considered to be new reagents for the palladium-catalyzed C–H activation reaction, which enables the transfer of methyl and other alkyl groups from the sulfonium salt to the aniline derivatives under mild catalytic conditions.
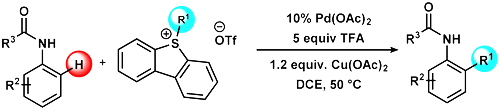
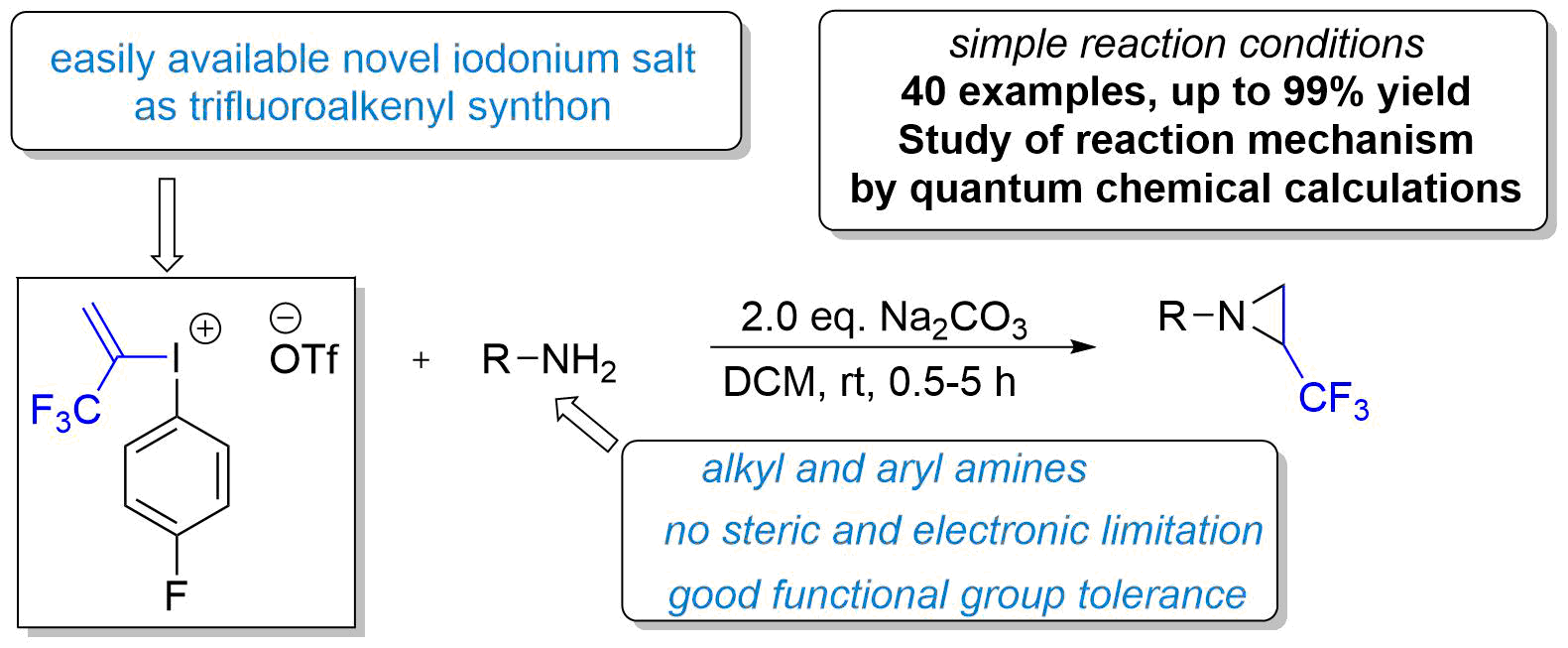
65. Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring
Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring, Ádám Mészáros, Anna Székely, András Stirling, Zoltán Novák, Angew. Chem. Int. Ed. 2018, 57, 6643-6647. DOI: 10.1002/anie.201802347 | [Full Text Link] [Supp. Info. Link]
Synthesis of fluorinated compounds and their use as pharmaceutical ingredients or synthetic building blocks are in the focus of chemical and medicinal research. However, the efficient synthesis of trifluoromethylated nitrogen heterocycles sometimes are challenging. Herein, we disclose a simple aziridination process which relies on the use of amines and novel alkenyl synthon for the access of trifluoromethylated strained heterocycle. With the utilization of a newly designed, bench stable but highly reactive hypervalent alkenyl iodonium species, the three membered heterocyclic ring can be constructed from simple amines without structural limitation with high efficiency under mild conditions in the absence of transition metal catalysts. The special reactivity of the new trifluoropropenyl synthon toward nucleophilic centres could be exploited in more general cyclization and alkenylation reactions in the future.
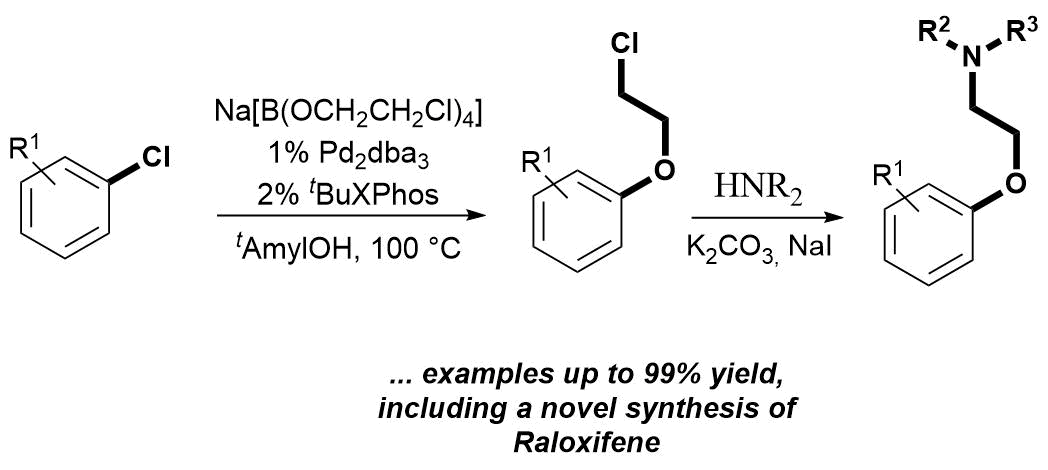
66. Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker
Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker, Bálint Pethő, Dóra Vangel, János Tivadar Csenki, Márton Zwillinger, Zoltán Novák, Org. Biomol. Chem. 2018. 16, 4895-4899. DOI: 10.1039/C8OB01146 | [Full Text Link] [Supp. Info. Link]
A novel disconnection based on cross-coupling chemistry was designed to access pharmaceutically relevant aryl-aminoethyl ethers. The developed palladium-catalyzed functionalization of aryl- and heteroaryl chlorides with sodium tetrakis-(2-chloroethoxy)-borate salt is orthogonal to the simple nucleophilic replacement of the chloro function of the ethylene linker. The transformation enables efficient 2-chloroethoxylation in the absence of additional external base. Subsequent amine substitution of the alkyl halide affords 2-aminoethoxy arenes. The applicability of this method was demonstrated through the synthesis of various aryl- and heteroaryl-alkyl ethers, including intermediates of marketed drug molecules.
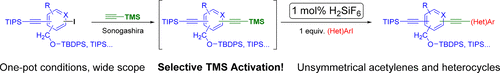
67. Catalytic Activation of Trimethylsilylacetylenes: A One-Pot Route to Unsymmetrical Acetylenes and Heterocycles
Catalytic Activation of Trimethylsilylacetylenes: A One-Pot Route to Unsymmetrical Acetylenes and Heterocycles, Dániel Lasányi, Ádám Mészáros, Zoltán Novák, Gergely L. Tolnai, J. Org. Chem. 2018. 83, 8281-8291. DOI: 10.1021/acs.joc.8b00998 | [Full Text Link] [Supp. Info. Link]
For the synthesis of unsymmetrical acetylenes, a Sonogashira coupling–deprotection–Sonogashira coupling reaction sequence is often used. Removal of protecting groups requires harsh conditions or an excess of difficult to handle and expensive reagents. Herein, we disclose a novel catalytic method for the selective deprotection of trimethylsilylacetylenes in Sonogashira reaction. The reagent hexafluorosilicic acid, an inexpensive nontoxic compound, was used to promote the selective desilylation. This method enables the efficient synthesis of unsymmetric acetylenes with other silylated functional groups present. Further possibilities of the method were explored by synthesis of heterocycles.
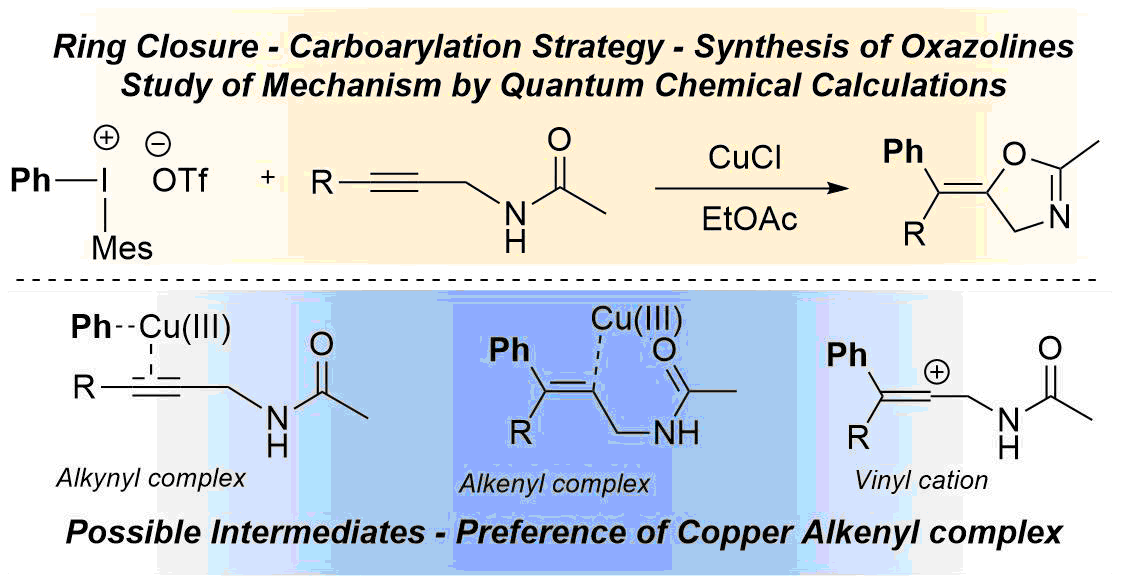
68. DFT Calculations on the Mechanism of Copper-Catalysed Tandem Arylation–Cyclisation Reactions of Alkynes and Diaryliodonium Salts
DFT calculations on the mechanism of copper-catalysed tandem arylation–cyclisation reactions of alkynes and diaryliodonium salts, Tamás Károly Stenczel, Ádám Sinai, Zoltán Novák, András Stirling. Beilstein. J. Org. Chem. 2018,14, 1743-1749. DOI: 10.3762/bjoc.14.148 | [Full Text Link] [Supp. Info. Link]
We present a computational mechanistic study on the copper(III)-catalysed carboarylation–ring closure reactions leading to the formation of functionalised heterocycles. We have performed DFT calculations along selected routes and compared their free energy profiles. The calculations considered two viable options for the underlying mechanism which differ in the order of the oxazoline ring formation and the aryl transfer steps. In our model transformation, it was found that the reaction generally features the aryl transfer–ring closing sequence and this sequence shows very limited sensitivity to the variation of the substituent of the reactants. On the basis of the mechanism the origin of the stereoselectivity is ascribed to the interaction of the Cu ion with the oxazoline oxygen driving the ring-closure step selectively.
Keywords: catalysis; DFT calculation; iodonium salts; reaction mechanism; tandem arylation–cyclisation