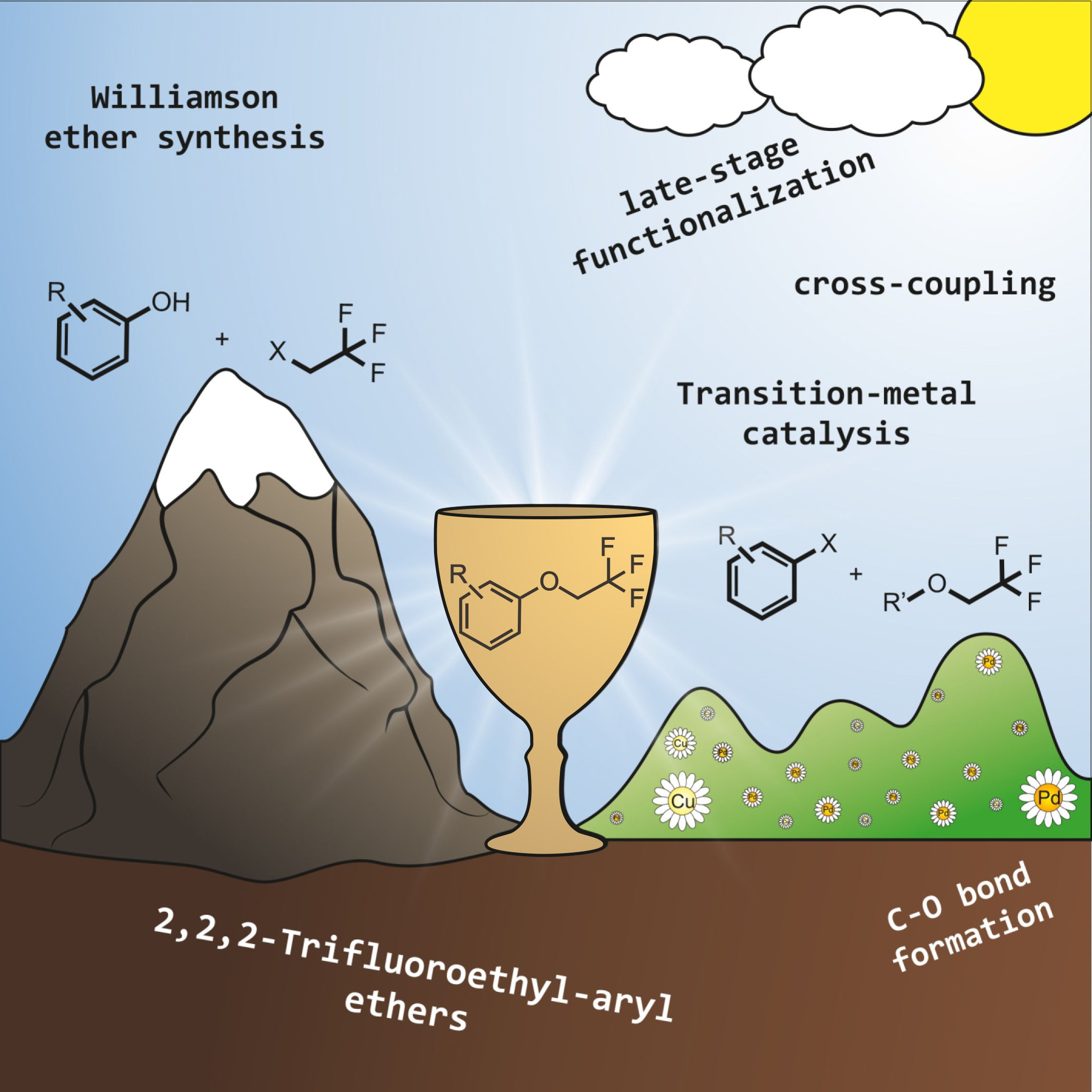
71. Synthesis of Multifunctional Aryl(trifloxyalkenyl)iodonium Triflate Salts
Synthesis of Multifunctional Aryl(trifloxyalkenyl)iodonium Triflate Salts, Balázs L. Tóth, Ferenc Béke, Orsolya Egyed, Attila Bényei, András Stirling, Zoltán Novák, ACS Omega 2019, 45, 9188-9197. DOI: 10.1021/acsomega.9b00728 | [Full Text Link] [Supp. Info. Link, CIF]
A convenient procedure for the synthesis of aryl(trifloxyalkenyl)iodonium triflate salts from commercially available (diacetoxyiodo)benzene, trimethylsilyl trifluoromethanesulfonate, and acetylenes under mild conditions was developed. The obtained multifunctional hypervalent vinyliodonium salts equipped with electrophilic and nucleophilic functions could serve as novel C2 synthons for organic transformations. The structure of the iodonium salts was identified by multidimensional NMR spectroscopy and X-ray crystallography.
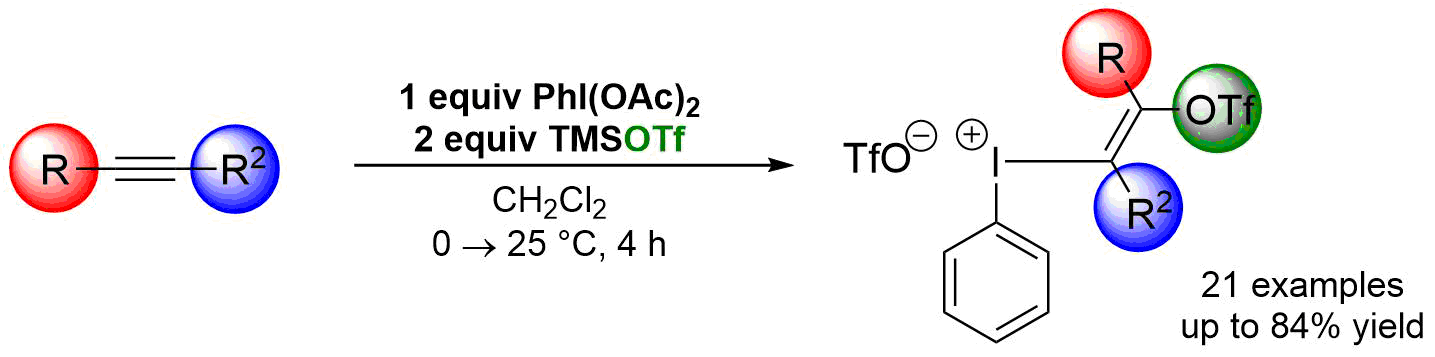
72. Design and application of diimine-based copper(I) complexes in photoredox catalysis
Design and application of diimine-based copper(I) complexes in photoredox catalysis, Tamás Földesi, Réka Adamik, Gellert Sipos, Bálint Nagy, Balázs L. Tóth, Attila Benyei, Krisztina Szekeres, Győző Láng, Attila Demeter, Timothy Peelen, Zoltán Novák, Org. Biomol. Chem. 2019, 17, 8343-8347. DOI: 10.1039/C9OB01331H | [Full Text Link] [Supp. Info. Link, CIF]
Structurally different bis(imino)copper(I) complexes were prepared in a highly modular manner and utilized as copper-based photocatalysts in ATRA reactions of styrenes and alkyl halides. The new photocatalysts showed good catalytic activity and ensured efficient chemical transformations.
Front cover of the issue: Org. Biomol. Chem.2019,17, 8263-8264. DOI:10.1039/C9OB90149C | [Full Text Link]

73. Efficient Synthesis of 3,4-Disubstituted 7-Azaindoles Employing SEM as a Dual Protecting–Activating Group
Efficient Synthesis of 3,4-Disubstituted 7-Azaindoles Employing SEM as a Dual Protecting–Activating Group, Piroska Gyárfás, János Gerencsér, Warren S. Wade, László Ürögdi, Zoltán Novák, S. Todd Meyer, Synlett 2019, 30, 2273-2278. DOI: 10.1055/s-0039-1690735 | [Full Text Link] [Supp. Info. Link]
An efficient method for nucleophilic aromatic substitution on 7-azaindoles has been developed. The reaction is facilitated by the unique dual influence of SEM as both protecting and activating group, permitting mild conditions and short reaction times that are compatible with sensitive functional groups. The method is suitable for the synthesis of a broad range of products, most notably ethers.
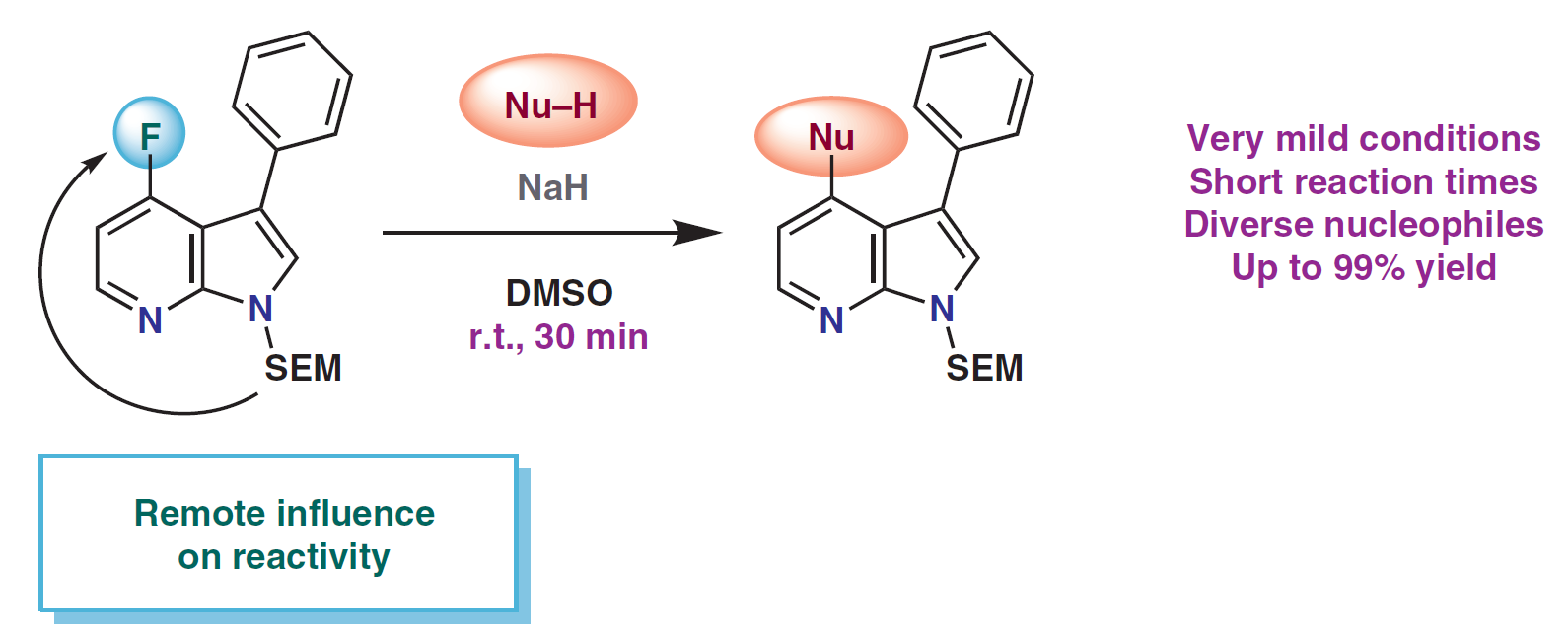
70. Synthesis of aryl‐ and heteroaryl‐trifluoroethyl ethers: aims, challenges and new methodologies
Synthesis of aryl‐ and heteroaryl‐trifluoroethyl ethers: aims, challenges and new methodologies, Bálint Pethő, Zoltán Novák, Asian J. Org. Chem. 2019, 568-575. DOI: 10.1002/ajoc.201800414 | [Full Text Link]
Focus Review: Fluorinated substances have outstanding importance in most fields of the chemical industry, from agrochemicals and material science to pharmaceutical research. Although aryl‐ and heteroaryl‐trifluoroethyl ethers have widespread applications, predominantly as drug molecules, their preparation was limited to classical synthetic methodologies until the last decade. The transition metal catalyzed cross coupling reactions offered novel synthetic approaches for the facile access of trifluoroethoxylated aromatic compounds. The aim of this review is to give a good overview on recent advances in the synthesis of aryl‐trifluoroethyl ethers from a practical point of view for synthetic organic chemists working in the field of pharmaceutical industry and academia.