42. Synthesis and Transformations of Oxygen Heterocycles
Synthesis and Transformations of Oxygen Heterocycles, Zoltán Novák, András Kotschy, Topics in Heterocyclic Chemistry Vol. 45, 231-303. DOI: 10.1007/7081_2014_136
The recent developments in the transition metal-catalyzed synthesis and transformations of such oxygen-containing heteroaromatic systems are reviewed, where the oxygen is part of a five-membered ring.
41. Mechanistic Study of Silver-Mediated Furan Formation by Oxidative Coupling
Mechanistic Study of Silver-Mediated Furan Formation by Oxidative Coupling, János Daru, Zsuzsanna Benda, Ádám Póti, Zoltán Novák, András Stirling, Chem. Eur. J. 2014, 20, 15395–15400. DOI: 10.1002/chem.201404302 | [Full Text Link] [Supp. Info Link]
Density functional calculations and experiments have been carried out to unravel the mechanism of a silver-mediated furan formation by oxidative coupling. Various possible reaction paths were considered and the most favorable channel has been identified on the basis of the calculated solvent-corrected Gibbs free-energy profiles. The mechanism represented by this route consists of a radical and a subsequent ionic route. The silver cation has a double role in the mechanism: it is the oxidant in the radical steps and the catalyst for the ionic steps, which is in accordance with the experimental observations. The two most important aspects of the optimal route are the formation of a silver–acetylide, reacting subsequently with the enolate radical, and the aromatic furan-ring formation in a single step at the latter, ionic segment of the reaction path. Our findings could explain several experimental observations, including the “key-promoter role” of silver, the preference for ionic cyclization, and the reduced reactivity of internal acetylides.
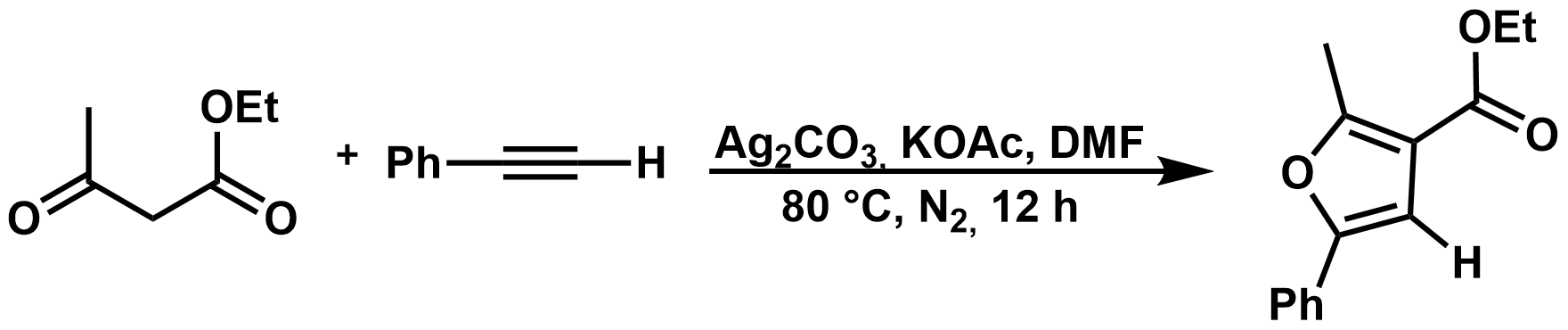
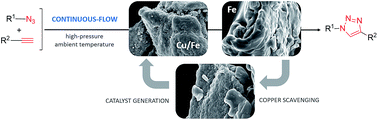
40. Continuous-flow azide–alkyne cycloadditions with an effective bimetallic catalyst and a simple scavenger system
Continuous-flow azide–alkyne cycloadditions with an effective bimetallic catalyst and a simple scavenger system, Sándor B. Ötvös, Gábor Hatoss, Ádám Georgiádes, Szabolcs Kovács, István M. Mándity, Zoltán Novák, Ferenc Fülöp, RSC Adv. 2014, 4, 46666-46674. DOI: 10.1039/C4RA07954J | [Supp. Info Link]
A flow chemistry-based technique is presented herein for Cu(I)-catalyzed azide–alkyne cycloadditions with a copper on iron bimetallic system as the catalyst and iron powder as a readily available copper scavenger. The method proved to be rapid and safe as compared with the conventional batch experiment; and by using an in-line copper scavenger, the level of copper impurities in the triazole products could readily be reduced to negligibly small amounts. The process was widely applicable, as not only terminal alkynes, but also various disubstituted acetylenes were nicely tolerated as dipolarophiles leading to useful 1,4,5-trisubstituted 1,2,3-triazoles.
39. Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and Heteroaromatic Iodides: The Beneficial Anchoring Effect of Borates
Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and Heteroaromatic Iodides: The Beneficial Anchoring Effect of Borates, Zsombor Gonda, Szabolcs Kovács, Csaba Wéber, Tamás Gáti, Attila Mészáros, András Kotschy, Zoltán Novák, Org. Lett. 2014, 16, 4268-4271. DOI: 10.1021/ol501967c | [Full Text Info] [Supp. Info Link, NMR]
Efficient copper-catalyzed trifluoromethylation of aromatic iodides was achieved with TMSCF3 in the presence of trimethylborate. The Lewis acid was used to anchor the in situ generated trifluoromethyl anion and suppress its rapid decomposition. Broad applicability of the new trifluoromethylating reaction was demonstrated in the functionalization of different aromatic and heteroaromatic iodides.
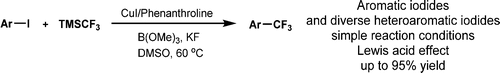
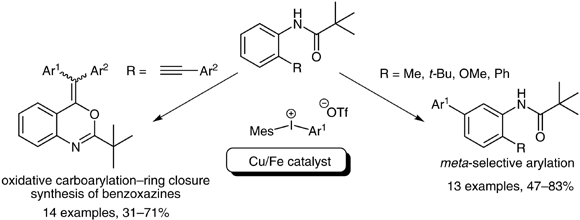
38. Utilization of a Copper on Iron Catalyst for the Synthesis of Biaryl Systems and Benzoxazines via Oxidative Arylation of Anilide Derivatives
Utilization of a Copper on Iron Catalyst for the Synthesis of Biaryl Systems and Benzoxazines via Oxidative Arylation of Anilide Derivatives, Anna Székely, Ádám Sinai, Edina B. Tóth, Zoltán Novák, Synthesis 2014, 14, 1871-1880. DOI: 10.1055/s-0033-1338642 | [Full Text Link] [Supp. Info Link]
Heterogeneous copper on iron catalyst serves as an efficient alternative copper source for arylation reactions using hypervalent iodonium salts. The copper(0) catalyst affords meta-arylation of pivalanilides, while 2-ethynylanilides undergo oxidative carboarylation–ring closure with diaryliodonium salts.
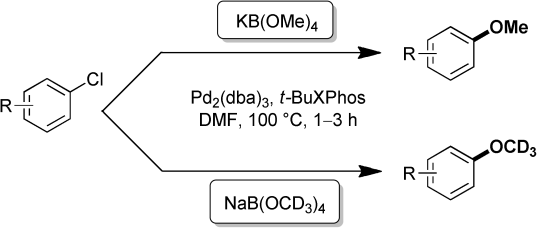
37. Palladium-Catalyzed Methoxylation of Aromatic Chlorides with Borate Salts
Palladium-Catalyzed Methoxylation of Aromatic Chlorides with Borate Salts, Gergely L. Tolnai, Bálint Pethő, Péter Králl, Zoltán Novák, Adv. Synth. Catal. 2014, 356, 125-129. DOI: 10.1002/adsc.201300687 | [Full Text Link] [Supp. Info Link]
Herein we disclose a simple palladium-catalyzed transformation for the methoxylation of aromatic chlorides with tetramethoxyborate salts. The procedure provides a new and efficient synthetic tool for the introduction of a methoxy group into aromatic systems. In addition, the reaction can be achieved using a wide range of aromatic and heteroaromatic chlorides, the cheapest class of halides.
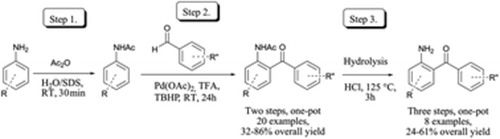
36. A one-pot process for palladium catalyzed direct C-H acylation of anilines in water using a removable ortho directing group
A one-pot process for palladium catalyzed direct C-H acylation of anilines in water using a removable ortho directing group ,Fruzsina Szabó, Dániel Simkó, Zoltán Novák, RSC Advances 2014, 4, 3883-3886. DOI: 10.1039/C3RA45160G | [Supp. Info Link]
A new mild, practical method for the synthesis of aminobenzophenone derivatives through a three step one-pot reaction sequence involving acylation of anilines, palladium catalyzed cross-dehydrogenative coupling of the formed anilides and the hydrolytic cleavage is reported. The full reaction sequence was performed under aqueous conditions.