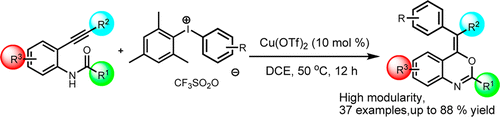
35. Copper-Catalyzed Oxidative Ring Closure and Carboarylation of 2-Ethynylanilides
Copper-Catalyzed Oxidative Ring Closure and Carboarylation of 2-Ethynylanilides, Ádám Sinai, Ádám Mészáros, Tamás Gáti, Veronika Kudar , Anna Palló, Zoltán Novák, Org. Lett. 2013, 15, 5654–5657. DOI: 10.1021/ol402600r | [Full Text Link] [Supp. Info Link, CIF1, CIF2]
A new copper-catalyzed oxidative ring closure of ethynyl anilides with diaryliodonium salts was developed for the highly modular construction of benzoxazines bearing a fully substituted exo double bond. The oxidative transformation includes an unusual 6-exo-dig cyclization step with the formation of C–O and C–C bonds.
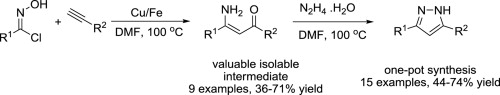
34. Copper on iron promoted one-pot synthesis of β-aminoenones and 3,5-disubstituted pyrazoles
Copper on iron promoted one-pot synthesis of β-aminoenones and 3,5-disubstituted pyrazoles, Szabolcs Kovács, Zoltán Novák, Tetrahedron 2013, 69, 8987–8993. DOI: 10.1016/j.tet.2013.08.047 |
The reaction of hydroximoyl chlorides with acetylenes in the presence of a copper on iron bimetallic system leads to β-aminoenones via reductive ring opening of isoxazole intermediates. The valuable β-aminoenone building blocks can be isolated or transformed into pyrazoles with the addition of hydrazine in a straightforward one-pot procedure.
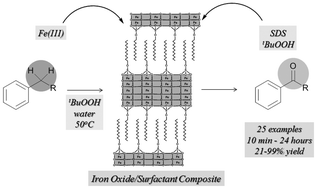
33. Mild Palladium-Catalyzed Oxidative Direct ortho C-H aczlation of Anilides under Aqueous Conditions
Mild Palladium-Catalyzed Oxidative Direct ortho C-H aczlation of Anilides under Aqueous Conditions, Fruzsina Szabó, János Daru, Dániel Simkó, Tibor Zs. Nagy, András Stirling, Zoltán Novák, Adv. Synth. Catal. 2013, 355, 685-691. DOI: 10.1002/adsc.201200948 | [Full Text Link] [Supp. Info Link]
Palladium-catalyzed cross-dehydrogenative coupling between anilides and aromatic aldehydes was achieved under aqueous conditions. A wide variety of the desired benzophenone derivatives was isolated in good to excellent yield. The reaction rate acceleration effect of acid and detergent has been demonstrated. Mechanistic insight has been obtained from quantum chemical calculations.
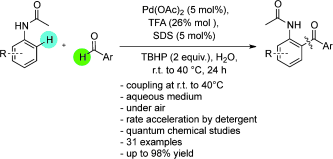
32. Iron-surfactant nanocomposite-catalyzed benzylic oxidation in water
Iron-surfactant nanocomposite-catalyzed benzylic oxidation in water, Fruzsina Szabó, Bálint Pethő, Zsombor Gonda, Zoltán Novák, RSC Advances 2013, 3, 4903-4908. DOI: 10.1039/C3RA22856H | [Supp. Info Link]
Benzylic oxidation in the presence of an iron–surfactant nanocomposite catalyst under aqueous conditions is described. A significant reaction rate acceleration in the presence of anionic surfactants was demonstrated. Several benzylic substrates were efficiently transformed to ketones under mild conditions.