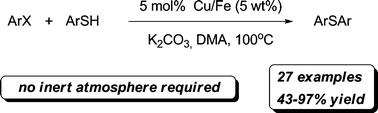
28. Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies
Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies, Douglas C. Behenna, Justin T. Mohr,Nathaniel H. Sherden, Smaranda C. Marinescu, Andrew M. Harned, Kousuke Tani, Masaki Seto, Sandy Ma, Zoltán Novák, Michael R. Krout, Ryan M. McFadden, Jennifer L. Roizen, John A. Enquist Jr., David E. White, Samantha R. Levine, Krastina V. Petrova, Akihiko Iwashita, Scott C. Virgil, Brian M. Stoltz, Chem. Eur. J. 2011, 17, 14199-14223. DOI: 10.1002/chem.201003383 | [Full Text Link] [Supp. Info Link]
α-Quaternary ketones are accessed through novel enantioselective alkylations of allyl and propargyl electrophiles by unstabilized prochiral enolate nucleophiles in the presence of palladium complexes with various phosphinooxazoline (PHOX) ligands. Excellent yields and high enantiomeric excesses are obtained from three classes of enolate precursor: enol carbonates, enol silanes, and racemic β-ketoesters. Each of these substrate classes functions with nearly identical efficiency in terms of yield and enantioselectivity. Catalyst discovery and development, the optimization of reaction conditions, the exploration of reaction scope, and applications in target-directed synthesis are reported. Experimental observations suggest that these alkylation reactions occur through an unusual inner-sphere mechanism involving binding of the prochiral enolate nucleophile directly to the palladium center.
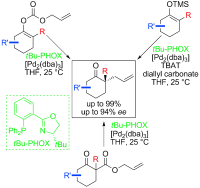
27. N-Halosuccinimide/SiCl4 as general, mild and efficient systems for the α-monohalogenation of carbonyl compounds and for benzylic halogenation
N-Halosuccinimide/SiCl4 as general, mild and efficient systems for the α-monohalogenation of carbonyl compounds and for benzylic halogenation, Tarek A. Salama, Zoltán Novák, Tetrahedron Lett. 2011, 52, 4026-4029. DOI: 10.1016/j.tetlet.2011.05.135 |
Combinations of N-halosuccinimide and tetrachlorosilane in acetonitrile were found to be efficient systems for the selective α-monohalogenation of carbonyl compounds as well as for benzylic halogenation under mild conditions.
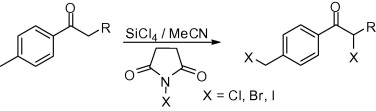
26. Oxidoreductive Coupling of Thiols with Aryl Halides Catalyzed by Copper on Iron
Oxidoreductive Coupling of Thiols with Aryl Halides Catalyzed by Copper on Iron, Szabolcs Kovács, Zoltán Novák, Org. Biomol. Chem. 2011, 9, 711-716. DOI: 10.1039/C0OB00397B | [Supp. Info Link]
Synthesis and utilization of a simple copper on iron catalyst in the coupling of aryl halides with thiols through disulfide intermediate is reported. The iron support of copper catalyst ensures reductive media for the coupling, allows easy removal of the metals by outer magnetic field and enables the recycling of the catalyst.