25. Efficient Synthesis of Deuterated 1,2,3-Triazoles
Efficient Synthesis of Deuterated 1,2,3-Triazoles, Zsombor Gonda, Krisztán Lőrincz, Zoltán Novák, Tetrahedron Lett. 2010, 51, 6275-6277. DOI: 10.1016/j.tetlet.2010.09.097 | [Full Text Link]
A wide variety of 1-monosubstituted 1,2,3-triazoles were synthesized efficiently via a copper-catalyzed click-reaction between azides and acetylene gas, generated in situ from CaC2 with the addition of H2O or D2O.
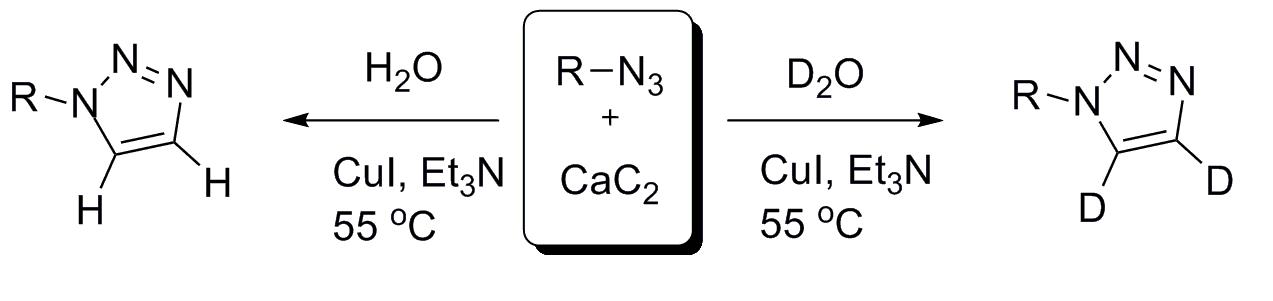
24. Dramatic Impact of ppb Levels of Palladium on the "Copper-Catalyzed" Sonogashira Coupling
Dramatic Impact of ppb Levels of Palladium on the "Copper-Catalyzed" Sonogashira Coupling, Zsombor Gonda, Gergely L. Tolnai, Zoltán Novák, Chem. Eur. J. 2010, 16, 11822-11826. DOI: 10.1002/chem.201001880 | [Full Text Link] [Supp. Info Link]
Palladacadabra! The effect of ppb levels of palladium on the “copper-catalyzed” Sonogashira coupling is reported. The observed high sensitivity to palladium impurities queries the existence of pure copper catalysis in the coupling of aryl iodides and terminal acetylenes (see figure).
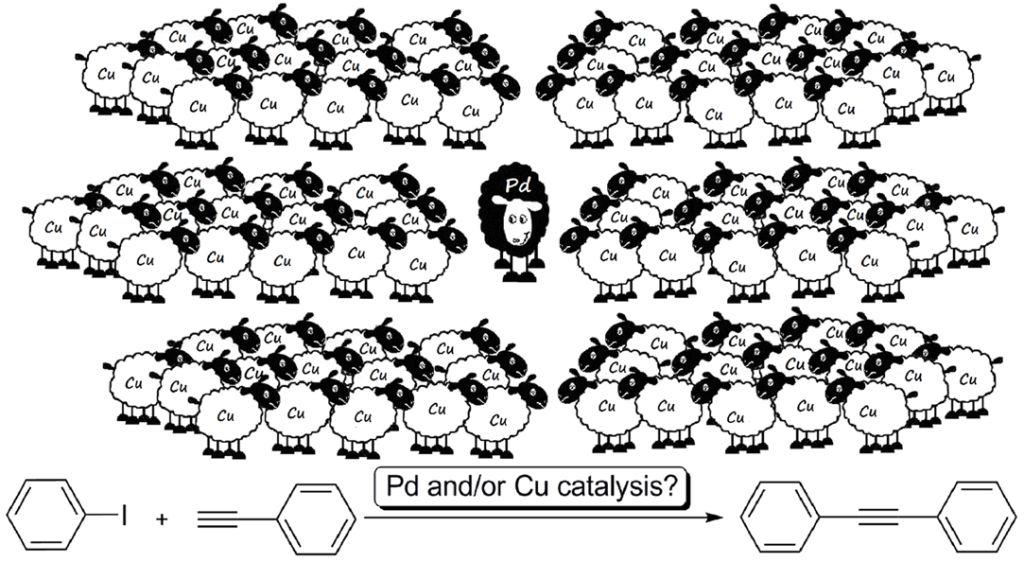
23. Activity of palladium on charcoal catalysts in cross-coupling reactions
Activity of palladium on charcoal catalysts in cross-coupling reactions, Anna Komáromi, Fruzsina Szabó, Zoltán Novák, Tetrahedron Lett. 2010, 51, 5411-5414. DOI: 10.1016/j.tetlet.2010.07.170 |
Comparison of the activity of several commercially available Pd/C catalysts in C–C, C–N, and C–S bond forming cross-coupling reactions has demonstrated the importance of the choice of the catalyst source. Investigations showed marked difference in activity between the catalysts. Moreover, the catalytic activity of each catalyst varies with respect to the coupling. The first Pd/C catalyzed Hiyama coupling is reported.
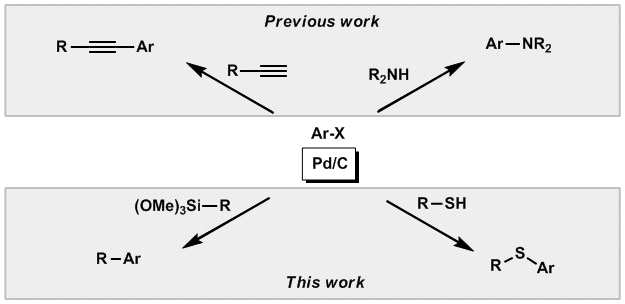
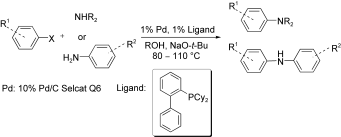
22. Examination of the Aromatic Amination Catalyzed by Palladium on Charcoal
Examination of the Aromatic Amination Catalyzed by Palladium on Charcoal, Anna Komáromi, Zoltán Novák, Adv. Synth. Catal. 2010, 352, 1523. DOI: 10.1002/adsc.201000048 | [Full Text Link] [Supp. Info Link]
The Buchwald–Hartwig amination of aryl halides with secondary amines and functionalized aromatic amines catalyzed by solid-supported palladium is reported. The choices of ligand, base and solvent are crucial for the successful coupling. The amination of aromatic iodides, bromides and chlorides can be easily achieved with palladium on charcoal in the presence of a biphenylphosphane-type ligand at 80–110 °C. In addition, the palladium on charcoal catalyst is easily separable after the reaction, and reusable several times with only small activity loss.
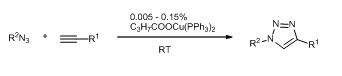
21. Highly Active Copper Catalysts for Azide-Alkyne Cycloaddition
Highly Active Copper Catalysts for Azide-Alkyne Cycloaddition, Zsombor Gonda, Zoltán Novák, Dalton Trans. 2010, 39, 726-729. DOI: 10.1039/b920790m | [Supp. Info Link]
Bis-triphenylphosphano complexes of copper(I)-carboxylates serve as efficient catalysts for azide-alkyne cycloaddition. The triazole formation takes place straightforwardly at ambient temperature providing a wide variety of products with good yields in the presence of 0.005–0.05% catalyst.