Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C-H Activation, Balázs L. Tóth, Szabolcs Kovács, Gergő Sályi, Zoltán Novák, Angew. Chem. Int. Ed. 2016, 55, 1988-1992. DOI: 10.1002/anie.201510555 | [Full Text Link] [Supp. Info. Link]
The introduction of trifluoroalkyl groups into aromatic molecules is an important transformation in the field of organic and medicinal chemistry. However, the direct installation of fluoroalkyl groups onto aromatic molecules still represents a challenging and highly demanding synthetic task. Herein, a simple trifluoroethylation process that relies on the palladium-catalyzed C-H activation of aromatic compounds is described. With the utilization of a highly active trifluoroethyl(mesityl)iodonium salt, the developed catalytic method enables the first highly efficient and selective trifluoroethylation of aromatic compounds. The robust catalytic procedure provides the desired products in up to 95 % yield at 25 °C in 1.5 to 3 hours and tolerates a broad range of functional groups. The utilization of hypervalent reagents opens new synthetic possibilities for direct alkylations and fluoroalkylations in the field of transition-metal-catalyzed C-H activation.
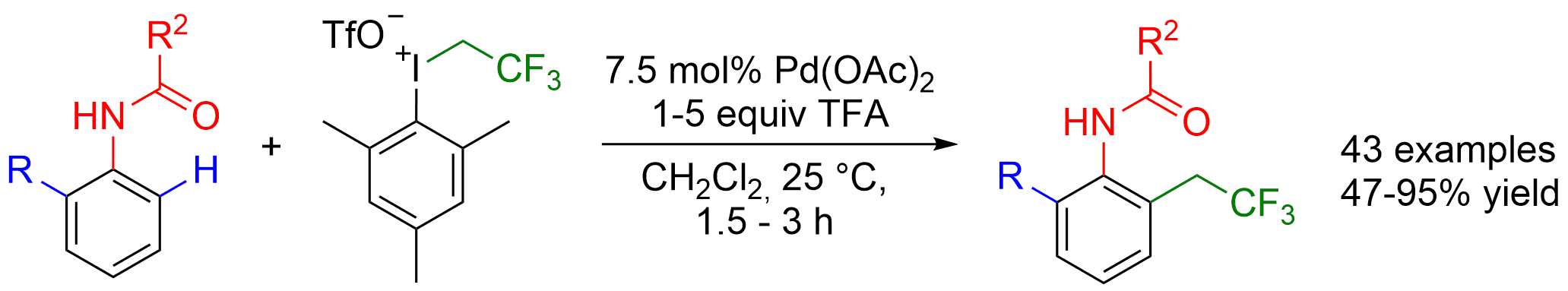
Simple: Anilides can be trifluoroethylated with a 2,2,2-trifluoroethyl-substituted iodonium salt in the presence of a palladium catalyst under mild conditions (see scheme). The reaction proceeds by CH activation at the ortho position and features a broad substrate scope.
Frontispiece: Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C−H Activation, DOI: 10.1002/anie.201680661
Trifluoroethylation In their Communication on page 1988 ff., Z. Novák and co-workers describe the use of a trifluoroethyl(mesityl)iodonium salt for the simple and efficient palladium-catalyzed trifluoroethylation of aromatic compounds by C−H activation.