Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N‑Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study, Tamás Bihari, Bence Babinszki, Zsombor Gonda, Szabolcs Kovács, Zoltán Novák, András Stirling, J. Org. Chem. 2016, 81, 5417-5422. DOI: 10.1021/acs.joc.6b00779 | [Full Text Link] [Supp. Info. Link]
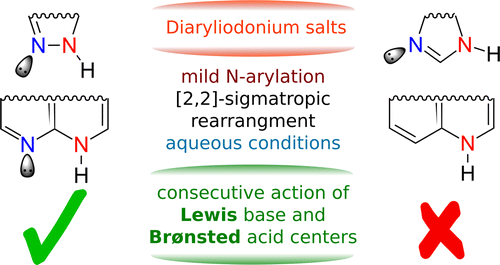
The mechanism of arylation of N-heterocycles with unsymmetric diaryliodonium salts is elucidated. The fast and efficient N-arylation reaction is interpreted in terms of the bifunctionality of the substrate: The consecutive actions of properly oriented Lewis base and Brønsted acid centers in sufficient proximity result in the fast and efficient N-arylation. The mechanistic picture points to a promising synthetic strategy where suitably positioned nucleophilic and acidic centers enable functionalization, and it is tested experimentally.