62. Copper-Catalyzed N-Arylation of Nitroenamines with Diaryliodonium Salts
Copper-Catalyzed N-Arylation of Nitroenamines with Diaryliodonium Salts, Klára Aradi, Ádám Mészáros, Balázs L. Tóth, Zoltán Vincze, Zoltán Novák, J. Org. Chem. 2017, 82, 11752-11764. DOI: 10.1021/acs.joc.7b01591 | [Full Text Link] [Supp. Info. Link]
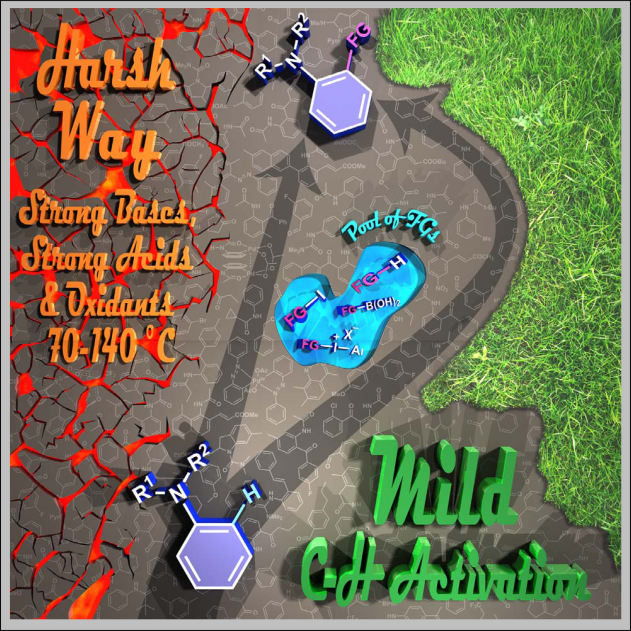
A novel synthetic methodology was developed for the N-arylation of nitroenamine derivatives utilizing diaryliodonium triflates and copper (I) chloride as a catalyst. The procedure enables the ease aryl transfer from the hypervalent species under mild catalytic conditions with unusual heteroatom preference and high efficiency.
63. Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil
Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil, Bálint Pethő, Márton Zwillinger, János Csenki, Anna Káncz, Balázs Krámos, Judit Müller, György Tibor Balogh, Zoltán Novák, Chem. Eur. J. 2017, 62, 15628-15632. DOI: 10.1002/chem.201704205 | [Full Text Link] [Supp. Info. Link]
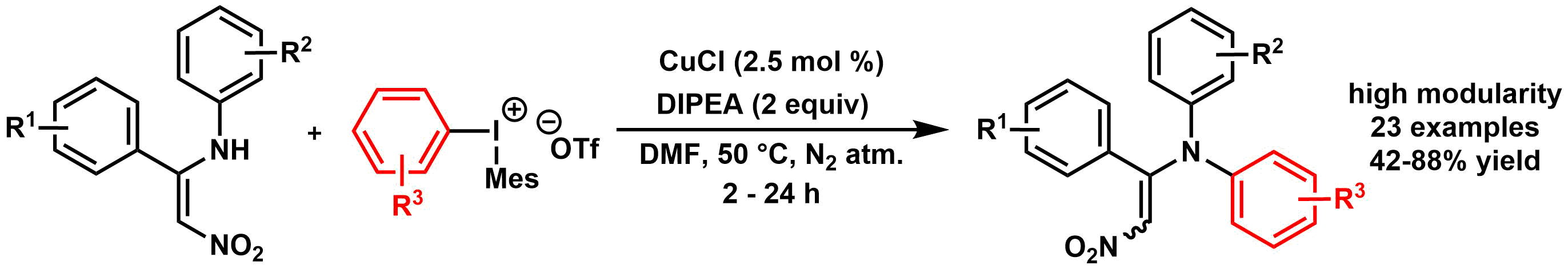
A simple and convenient method was developed for the introduction of 2,2,2-trifluoroethoxy group to various aromatic and heteroaromatic systems. The novel process utilizes aromatic chlorides as substrates, and tetrakis(2,2,2-trifluoroethoxy) borate salt as an inexpensive and readily available fluoroalkoxy source in a palladium-catalyzed cross-coupling reaction. The power of the developed methodology was demonstrated in the synthesis of fluorous derivative of Sildenafil.
56. Study of Lewis Acid Accelerated Palladium Catalyzed C-H Activation
Study of Lewis Acid Accelerated Palladium Catalyzed C-H Activation, Orsolya Tischler, Szabolcs Kovács, Gábor Érsek, Péter Králl, János Daru, András Stirling, Zoltán Novák, J. Mol. Catal. Chem. 2017, 426, 444-450. DOI: 10.1016/j.molcata.2016.09.018
This paper is dedicated to Professor Georgiy B. Shul’pin on the occasion of his 70th birthday.
Keywords
C-H activation; palladium; Lewis acid; DFT studies
Highlights
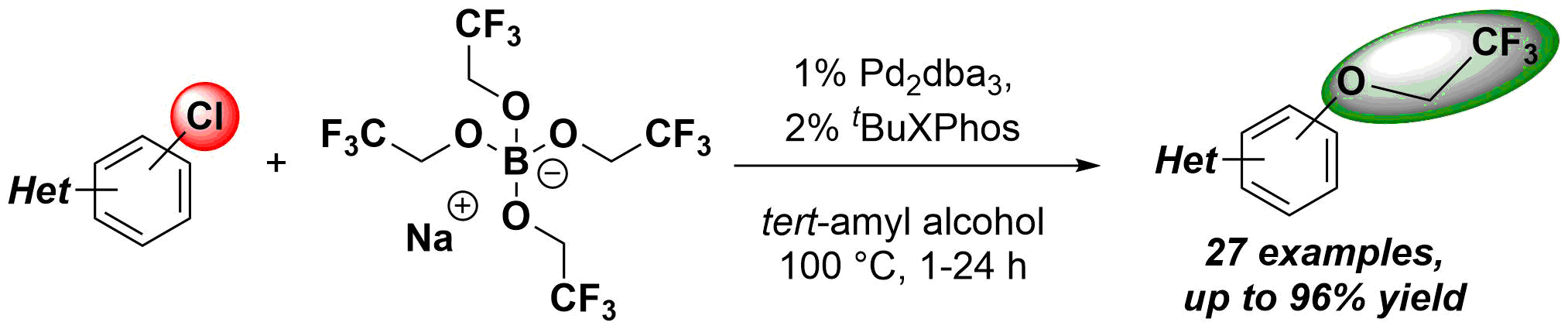
•Amide and urea directed ortho-acylation and ortho-olefination on aromatic cores is accelerated by Lewis acidic additives.
•Lewis acidic additives afford more electrophilic transition metal center, favoring C-H activation.
•The efficiency of several palladium(II) catalysts is raised.
•KIE experiments and calculations suggests a rate-determining C-H activation step.
Abstract
Acceleration of palladium catalyzed C-H activation by various Lewis Acids was demonstrated on the directed ortho-alkenylation and acylation of acetanilide and urea derivatives. The universality of this effect was investigated by the study of different palladium catalysts, directing groups in the aromatic substrates and versatile Lewis acids. Experiments were carried out to monitor the reactions and to compare the behavior and activity of different types of Lewis acids. Kinetic investigation revealed a rate determining C-H activation step, and DFT studies were performed for the explanation of Lewis acid effect on C-H activation.
55. Mild Palladium Catalyzed ortho C-H Bond Functionalizations of Aniline Derivatives
Mild Palladium Catalyzed ortho C-H Bond Functionalizations of Aniline Derivatives, Orsolya Tischler, Balázs L. Tóth, Zoltán Novák, Chem. Rec. 2017, 17, 184-199. DOI: 10.1002/tcr.201600059 | [Full Text Link]
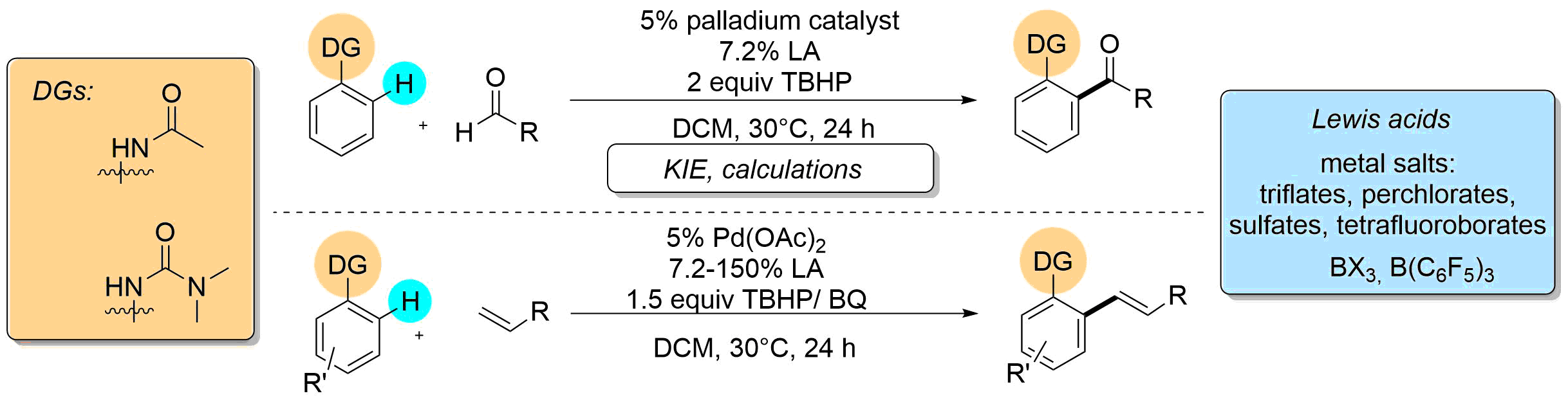
This account collects the developments and transformations which avoid the utilization of harsh reaction conditions in the field of palladium catalyzed, ortho-directed C-H activation of aniline derivatives from the first attempts to up-to-date results, including the results of our research laboratory. The discussed functionalizations performed under mild conditions include acylation, olefination, arylation, alkylation, alkoxylation reactions. Beside the optimization studies and the synthetic applications mechanistic investigations are also presented.