Study of Lewis Acid Accelerated Palladium Catalyzed C-H Activation, Orsolya Tischler, Szabolcs Kovács, Gábor Érsek, Péter Králl, János Daru, András Stirling, Zoltán Novák, J. Mol. Catal. Chem. 2017, 426, 444-450. DOI: 10.1016/j.molcata.2016.09.018
This paper is dedicated to Professor Georgiy B. Shul’pin on the occasion of his 70th birthday.
Keywords
C-H activation; palladium; Lewis acid; DFT studies
Highlights
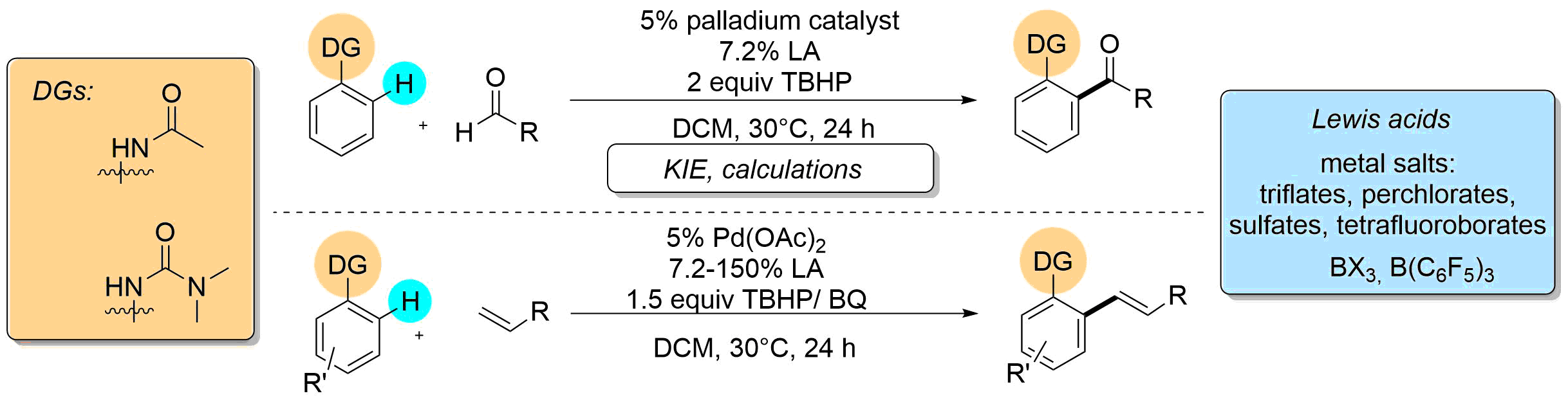
•Amide and urea directed ortho-acylation and ortho-olefination on aromatic cores is accelerated by Lewis acidic additives.
•Lewis acidic additives afford more electrophilic transition metal center, favoring C-H activation.
•The efficiency of several palladium(II) catalysts is raised.
•KIE experiments and calculations suggests a rate-determining C-H activation step.
Abstract
Acceleration of palladium catalyzed C-H activation by various Lewis Acids was demonstrated on the directed ortho-alkenylation and acylation of acetanilide and urea derivatives. The universality of this effect was investigated by the study of different palladium catalysts, directing groups in the aromatic substrates and versatile Lewis acids. Experiments were carried out to monitor the reactions and to compare the behavior and activity of different types of Lewis acids. Kinetic investigation revealed a rate determining C-H activation step, and DFT studies were performed for the explanation of Lewis acid effect on C-H activation.