Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions, Szabolcs Kovács, Balázs L. Tóth, Gábor Borsik, Tamás Bihari, Nóra V. May, András Stirling, Zoltán Novák, Adv. Synth. Catal. 2017, 359, 527-532. DOI: 10.1002/adsc.201601136 | [Full Text Link] [Supp. Info. Link]
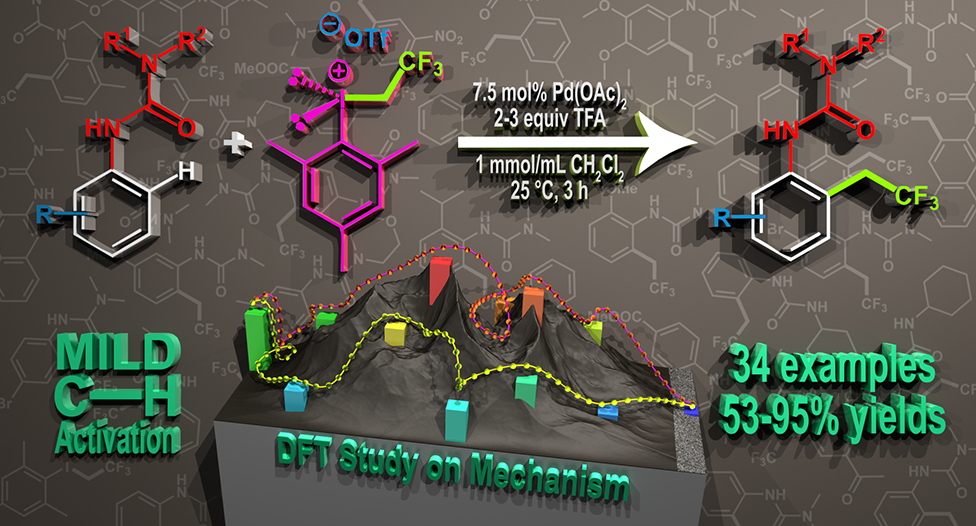
Abstract: Development of direct late-stage installation of alkyl groups into aromatic systems is an important and challenging task of current organic chemistry. In spite of the existing functionalization methods in organic chemistry for the substitution reactions on aromatic systems, the direct alkylation of aromatic ureas is unknown. Herein, as a first example we report a novel palladium catalyzed fluoroalkylation process by C−H activation for the access of ortho trifluoroethylated aromatic ureas. The application of novel, highly active trifluoroethyl(mesityl)iodonium salt enables the efficient introduction of the trifluoroethyl group at 25 °C in 3 hours in high yields (up to 95%) with good functional group tolerance. DFT calculations have revealed a rate determining oxidative alkyl-group transfer preceded by an unexpected C−H activation route on the Pd center during the catalytic cycle, where the deprotonation is assisted by an external triflate anion.