Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil, Bálint Pethő, Márton Zwillinger, János Csenki, Anna Káncz, Balázs Krámos, Judit Müller, György Tibor Balogh, Zoltán Novák, Chem. Eur. J. 2017, 62, 15628-15632. DOI: 10.1002/chem.201704205 | [Full Text Link] [Supp. Info. Link]
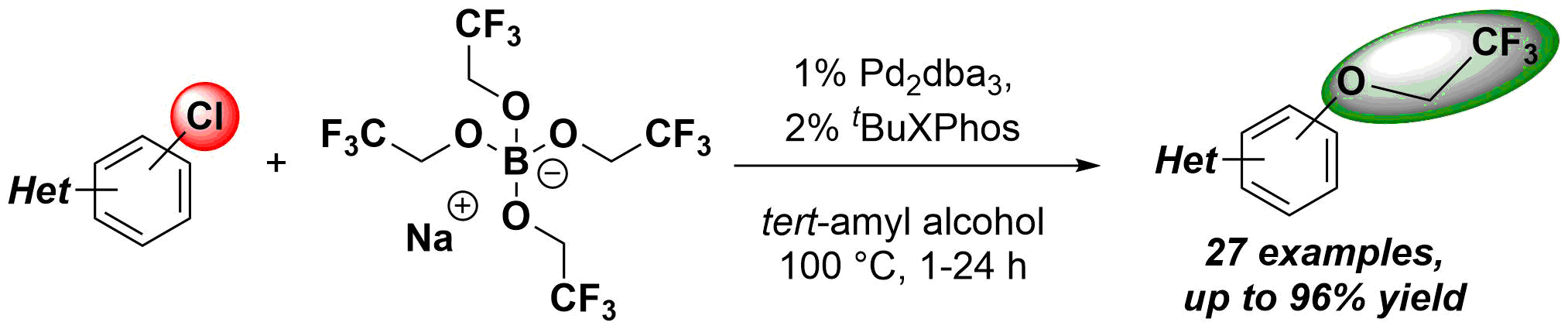
A simple and convenient method was developed for the introduction of 2,2,2-trifluoroethoxy group to various aromatic and heteroaromatic systems. The novel process utilizes aromatic chlorides as substrates, and tetrakis(2,2,2-trifluoroethoxy) borate salt as an inexpensive and readily available fluoroalkoxy source in a palladium-catalyzed cross-coupling reaction. The power of the developed methodology was demonstrated in the synthesis of fluorous derivative of Sildenafil.