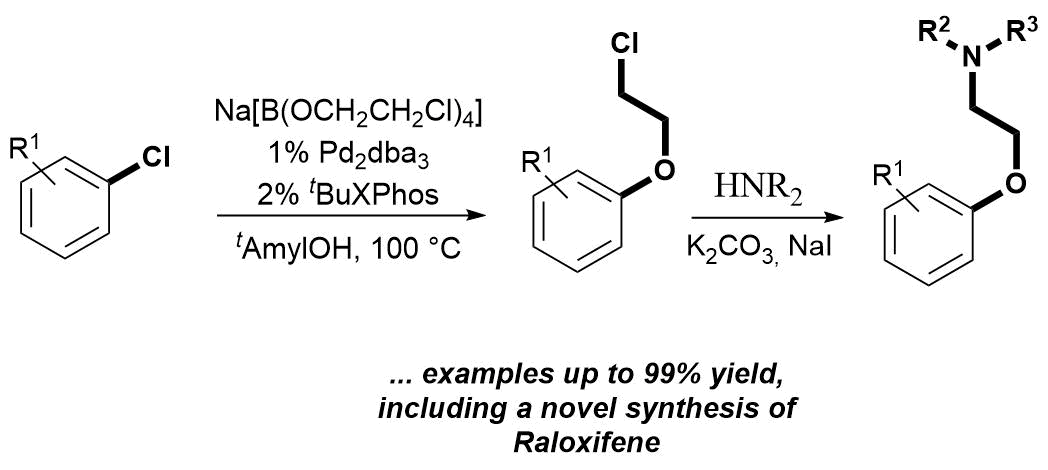
Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker, Bálint Pethő, Dóra Vangel, János Tivadar Csenki, Márton Zwillinger, Zoltán Novák, Org. Biomol. Chem. 2018. 16, 4895-4899. DOI: 10.1039/C8OB01146 | [Full Text Link] [Supp. Info. Link]
A novel disconnection based on cross-coupling chemistry was designed to access pharmaceutically relevant aryl-aminoethyl ethers. The developed palladium-catalyzed functionalization of aryl- and heteroaryl chlorides with sodium tetrakis-(2-chloroethoxy)-borate salt is orthogonal to the simple nucleophilic replacement of the chloro function of the ethylene linker. The transformation enables efficient 2-chloroethoxylation in the absence of additional external base. Subsequent amine substitution of the alkyl halide affords 2-aminoethoxy arenes. The applicability of this method was demonstrated through the synthesis of various aryl- and heteroaryl-alkyl ethers, including intermediates of marketed drug molecules.