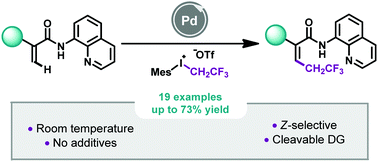
Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature, Louise Ruyet, Maria I. Lapuh, Vijay S. Koshti, Tamás Földesi, Philippe Jubault, Thomas Poisson, Zoltán Novák, Tatiana Besset, Chem. Commun. 2021, 57, 6241-6244. DOI: 10.1039/D1CC02007B | [Supp. Info. Link]
A straightforward 2,2,2-trifluoroethylation of acrylamides by Pd-catalyzed C–H bond activation was reported by using a fluorinated hypervalent iodine reagent as a coupling partner. At room temperature, this additive-free approach allowed the synthesis of Z-2,2,2-trifluoroethylated acrylamides (19 examples, up to 73% yield) in a stereoselective manner. Under these mild reaction conditions, the methodology turned out to be functional group tolerant and mechanistic studies gave us a better understanding of the transformation.