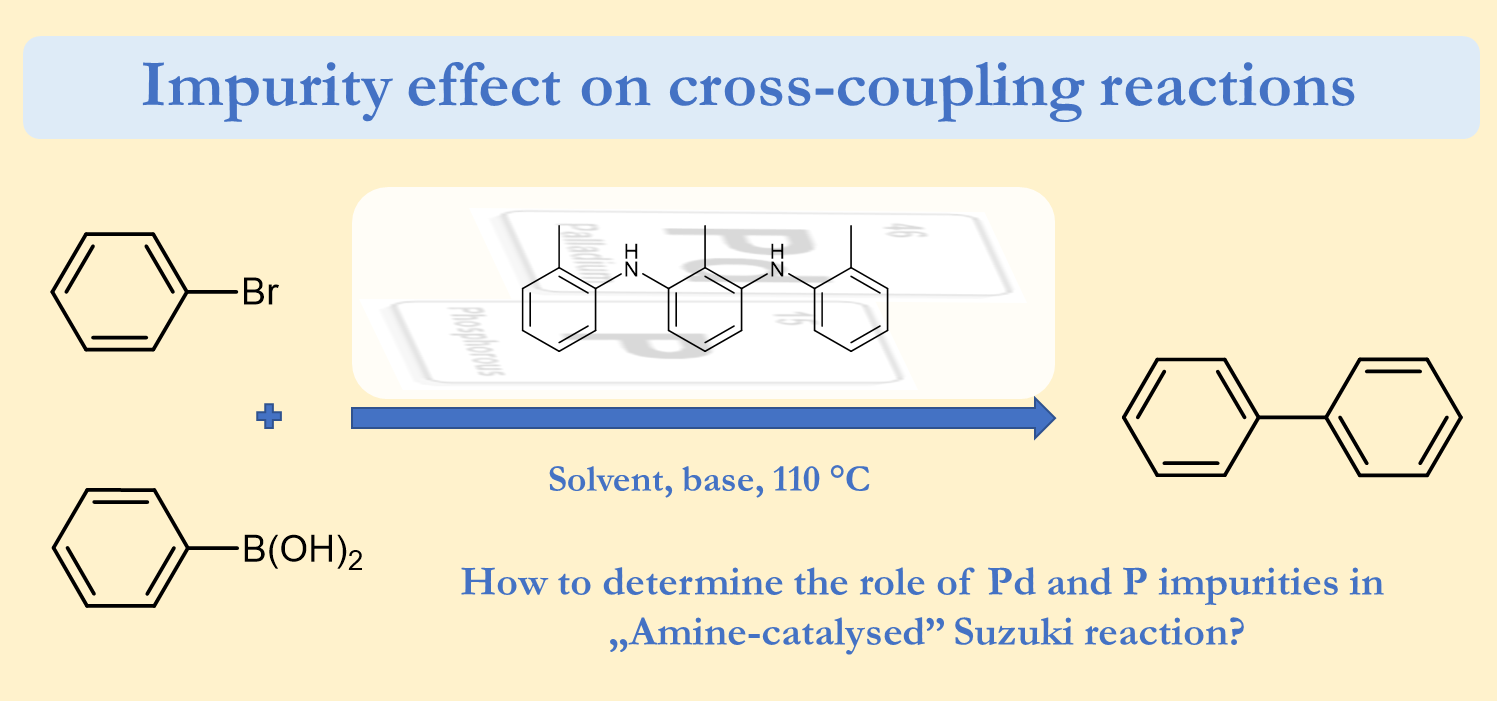
Revisiting the amine-catalysed cross-coupling, Zoltán Novák, Réka Adamik, János T. Csenki, Ferenc Béke, Regina Gavaldik, Bálint Varga, Bálint Nagy, Zoltán May, János Daru, Zsombor Gonda, Gergely L. Tolnai, Nature Catal. 2021, 4, 991–993. DOI: 10.1038/s41929-021-00709-8 [Supp. Info. Link] [Supp. Info. Link 2] [ChemRxiv PrePrint]
Several efforts have been made for the replacement of noble metal palladium in cross-coupling reactions, maintaining high efficiency of the target transformation. In several cases it is possible to perform the chemistry of palladium with related metals, and their activity was supported with mechanistic studies. Moreover, the complete exclusion of Pd is also in focus. Very recently it was demonstrated that special amine organocatalysts could catalyse Suzuki-Miyaura coupling reaction. Here we show that in this recent transformation homeopathic palladium impurities and trace phosphorous species originated from the conditions used for the organocatalyst synthesis are responsible for the catalytic effect instead of the amine species. This finding confirms the power of palladium in cross-coupling and draw the attention of impurity effect in this field of chemical research. In this article, we represent general guidelines for elucidating the real catalyst of reactions.