Synthesis of Hydrofluoroolefin-Based Iodonium Reagent via Dyotropic Rearrangement and Its Utilization in Fluoroalkylation, János T. Csenki, Balázs L. Tóth, Ferenc Béke, Bálint Varga, Péter P. Fehér, András Stirling, Zsuzsanna Czégény, Attila Bényei, Zoltán Novák, Angew. Chem. Int. Ed. 2022, 61, ASAP. DOI: 10.1002/anie.202208420 | [Full Text Link] [Supp. Info Link] [Crystal Data (CIF)]
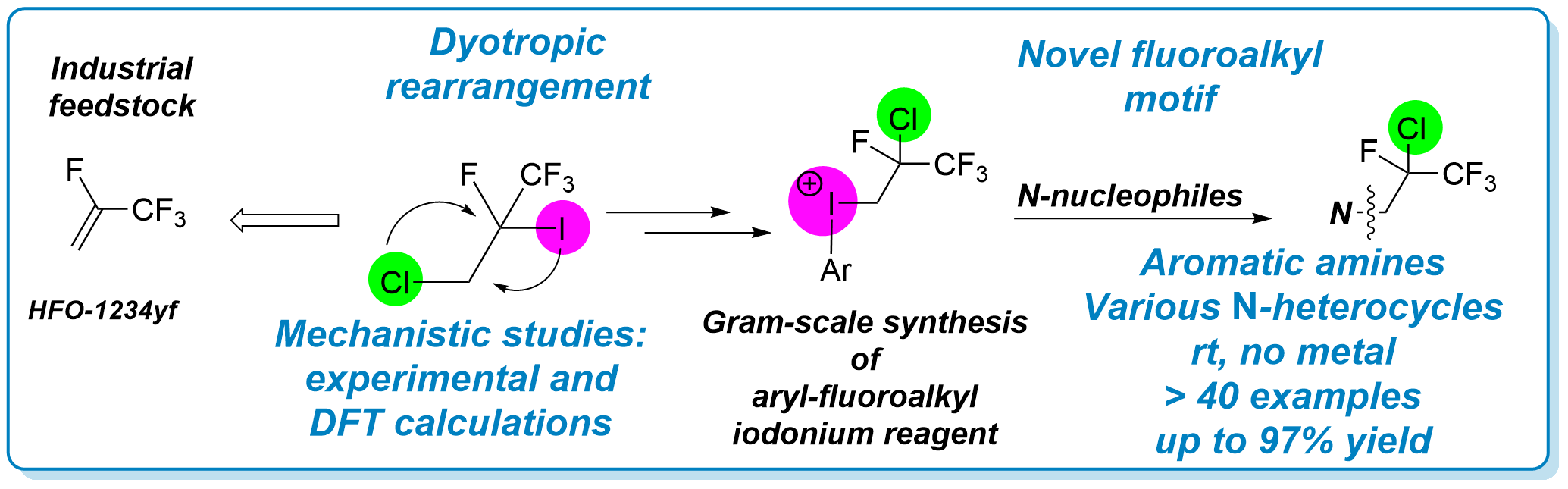
The synthesis of a novel hypervalent iodonium salt was developed with the exploitation of a dyotropic rearrangement of iodine and chlorine functions in fluoroalkyl chains from HFO-1234yf gas. The electrophilic reagent enabled the direct and metal-free introduction of a special fluoroalkyl group into aniline derivatives and N-containing heteroaromatic compounds. Detailed experimental and computational data were collected and used to propose a mechanistic description of this unique rearrangement.