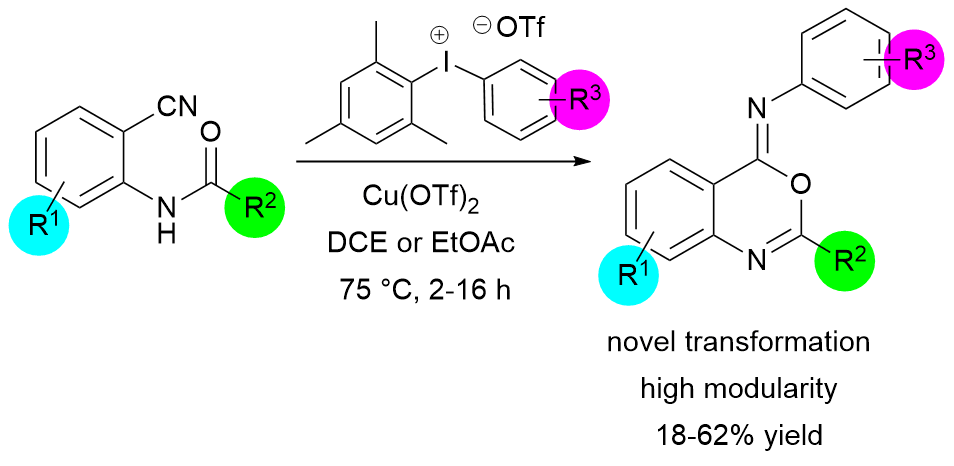
47. Transition-Metal-Free N-Arylation of Pyrazoles with Diaryliodonium Salts
Transition-Metal-Free N-Arylation of Pyrazoles with Diaryliodonium Salts, Zsombor Gonda, Zoltán Novák, Chem. Eur. J. 2015, 21, 16801-16806. DOI: 10.1002/chem.201502995 | [Full Text Link] [Supp. Info. Link]
A new synthetic method was developed for the N-arylation of pyrazoles using diaryliodonium salts. The transformation does not require any transition-metal catalyst and provides the desired N-arylpyrazoles rapidly under mild reaction condition in the presence of aqueous ammonia solution as a mild base without the use of inert atmosphere. The chemoselectivity of unsymmetric diaryliodonium salts was also explored with large number of examples.
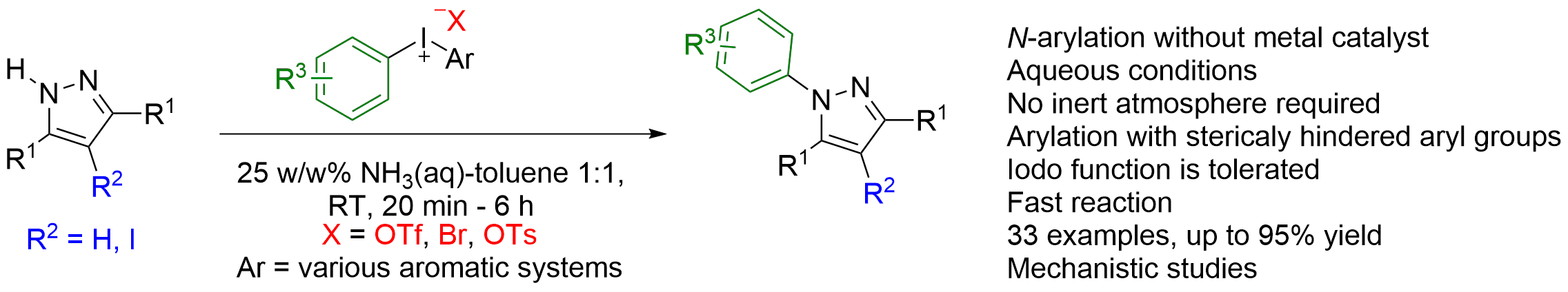
46. Utilization of Copper-Catalyzed Carboarylation–Ring Closure for the Synthesis of New Oxazoline Derivatives
Utilization of Copper-Catalyzed Carboarylation–Ring Closure for the Synthesis of New Oxazoline Derivatives, Ádám Sinai, Dóra Vangel, Tamás Gáti, Petra Bombicz, Zoltán Novák, Org. Lett. 2015, 17, 4136-4139. DOI: 10.1021/acs.orglett.5b01860 | [Full Text Link] [Supp. Info. Link, CIF]
A copper-catalyzed carboarylation–ring-closure strategy was used for the modular synthesis of oxazolines via the reaction of 1-aryl- and 1-alkylpropargylamides and diaryliodonium salts. The novel approach enables the efficient, modular synthesis of oxazoline derivatives bearing fully substituted exo double bonds.
45. Multistep Continuous-Flow Synthesis of Condensed Benzothiazoles
Multistep Continuous-Flow Synthesis of Condensed Benzothiazoles, Klára Lövei, István Greiner, János Éles, Áron Szigetvári, Miklós Dékány, Sándor Lévai, Zoltán Novák, György István Túrós, J. Flow Chem. 2015, 5, 74–81. DOI: 10.1556/1846.2015.00004 | [Full Text Link] [Supp. Info Link]
In medicinal chemistry, the development of synthetic procedures for the access of new heterocyclic systems as potential scaffolds is elementary. Herein, we report our results on the formation of small drug-like heterocycles, utilizing flow chemistry. This approach enables the extension of the reaction parameter window, including high-pressure/high-temperature or hazardous chemistry. In our work, various novel condensed tricyclic benzothiazoles fused with furo- and thieno-rings were synthesized applying a multistep continuous-flow protocol. The process includes two ring closure steps and a nitro group reduction step. Batch and telescoped continuous-flow syntheses were also designed and performed.
44. Efficient Direct 2,2,2-Trifluoroethylation of Indoles via C-H Functionalization
Efficient Direct 2,2,2-Trifluoroethylation of Indoles via C-H Functionalization, Gergely László Tolnai, Anna Székely, Zita Makó, Tamás Gáti, János Daru, Tamás Bihari, Andras Stirling and Zoltán Novák, Chem. Commun. 2015, 51, 4488-4491. DOI: 10.1039/C5CC00519A | [Supp. Info Link]
A novel highly C3 selective metal free trifluoroethylation of indoles using 2,2,2-trifuoroethyl(mesityl)-iodonium triflate was developed. The methodology enables the introduction of a trifluoroethyl group in a fast and efficient reaction under mild conditions with high functional group tolerance. Beyond the synthetic developments, quantum chemical calculations provide a deeper understanding of the transformation.
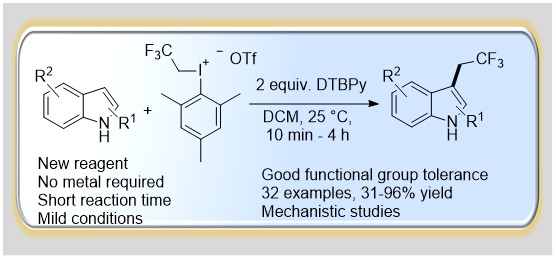
43. Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines
Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines, Klára Aradi, Zoltán Novák, Adv. Synt. Catal. 2015, 357, 371-376. DOI: 10.1002/adsc.201400763 | [Full Text Link] [Supp. Info Link]
A novel, highly modular synthetic methodology with high functional group tolerance was developed for the construction of iminobenzoxazine derivatives from ortho-cyanoanilides and diaryliodonium triflates via an oxidative arylation–cyclization path. The reaction is supposed to involve the formation of highly active aryl-copper(III) species. In this novel transformation, copper(II) triflate was used as catalyst in 1,2-dichloroethane or ethyl acetate and the reaction takes place at 75 °C in 2–16 h.