Continuous-flow azide–alkyne cycloadditions with an effective bimetallic catalyst and a simple scavenger system, Sándor B. Ötvös, Gábor Hatoss, Ádám Georgiádes, Szabolcs Kovács, István M. Mándity, Zoltán Novák, Ferenc Fülöp, RSC Adv. 2014, 4, 46666-46674. DOI: 10.1039/C4RA07954J | [Supp. Info Link]
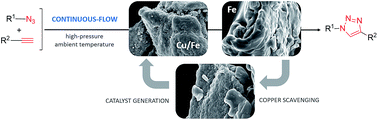
A flow chemistry-based technique is presented herein for Cu(I)-catalyzed azide–alkyne cycloadditions with a copper on iron bimetallic system as the catalyst and iron powder as a readily available copper scavenger. The method proved to be rapid and safe as compared with the conventional batch experiment; and by using an in-line copper scavenger, the level of copper impurities in the triazole products could readily be reduced to negligibly small amounts. The process was widely applicable, as not only terminal alkynes, but also various disubstituted acetylenes were nicely tolerated as dipolarophiles leading to useful 1,4,5-trisubstituted 1,2,3-triazoles.