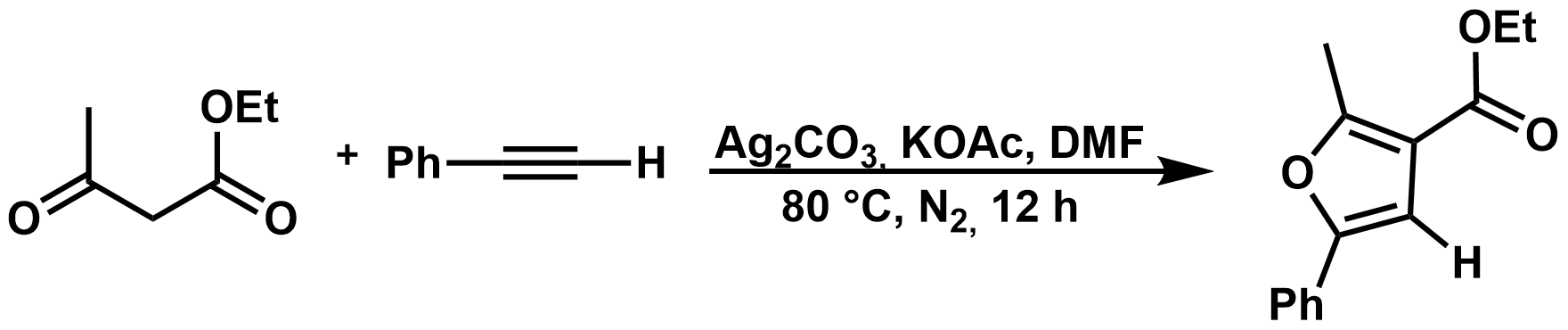Mechanistic Study of Silver-Mediated Furan Formation by Oxidative Coupling, János Daru, Zsuzsanna Benda, Ádám Póti, Zoltán Novák, András Stirling, Chem. Eur. J. 2014, 20, 15395–15400. DOI: 10.1002/chem.201404302 | [Full Text Link] [Supp. Info Link]
Density functional calculations and experiments have been carried out to unravel the mechanism of a silver-mediated furan formation by oxidative coupling. Various possible reaction paths were considered and the most favorable channel has been identified on the basis of the calculated solvent-corrected Gibbs free-energy profiles. The mechanism represented by this route consists of a radical and a subsequent ionic route. The silver cation has a double role in the mechanism: it is the oxidant in the radical steps and the catalyst for the ionic steps, which is in accordance with the experimental observations. The two most important aspects of the optimal route are the formation of a silver–acetylide, reacting subsequently with the enolate radical, and the aromatic furan-ring formation in a single step at the latter, ionic segment of the reaction path. Our findings could explain several experimental observations, including the “key-promoter role” of silver, the preference for ionic cyclization, and the reduced reactivity of internal acetylides.