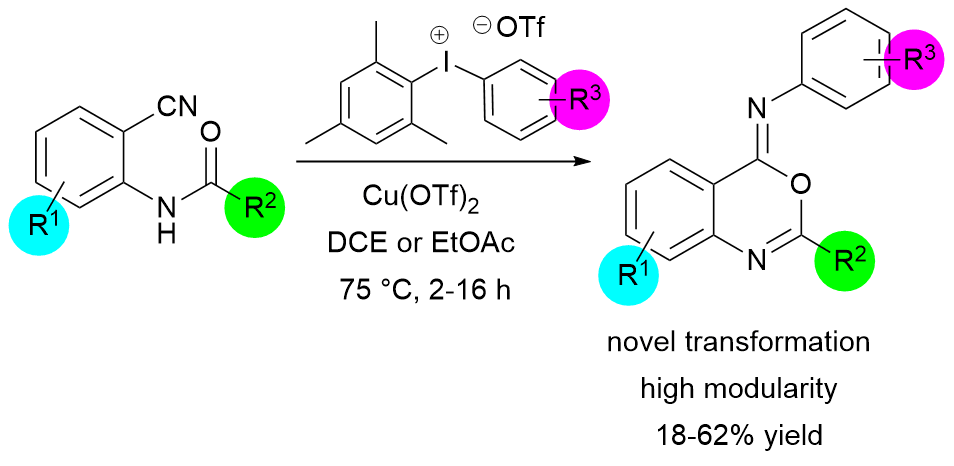
Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines, Klára Aradi, Zoltán Novák, Adv. Synt. Catal. 2015, 357, 371-376. DOI: 10.1002/adsc.201400763 | [Full Text Link] [Supp. Info Link]
A novel, highly modular synthetic methodology with high functional group tolerance was developed for the construction of iminobenzoxazine derivatives from ortho-cyanoanilides and diaryliodonium triflates via an oxidative arylation–cyclization path. The reaction is supposed to involve the formation of highly active aryl-copper(III) species. In this novel transformation, copper(II) triflate was used as catalyst in 1,2-dichloroethane or ethyl acetate and the reaction takes place at 75 °C in 2–16 h.