OPR&D Picked Up
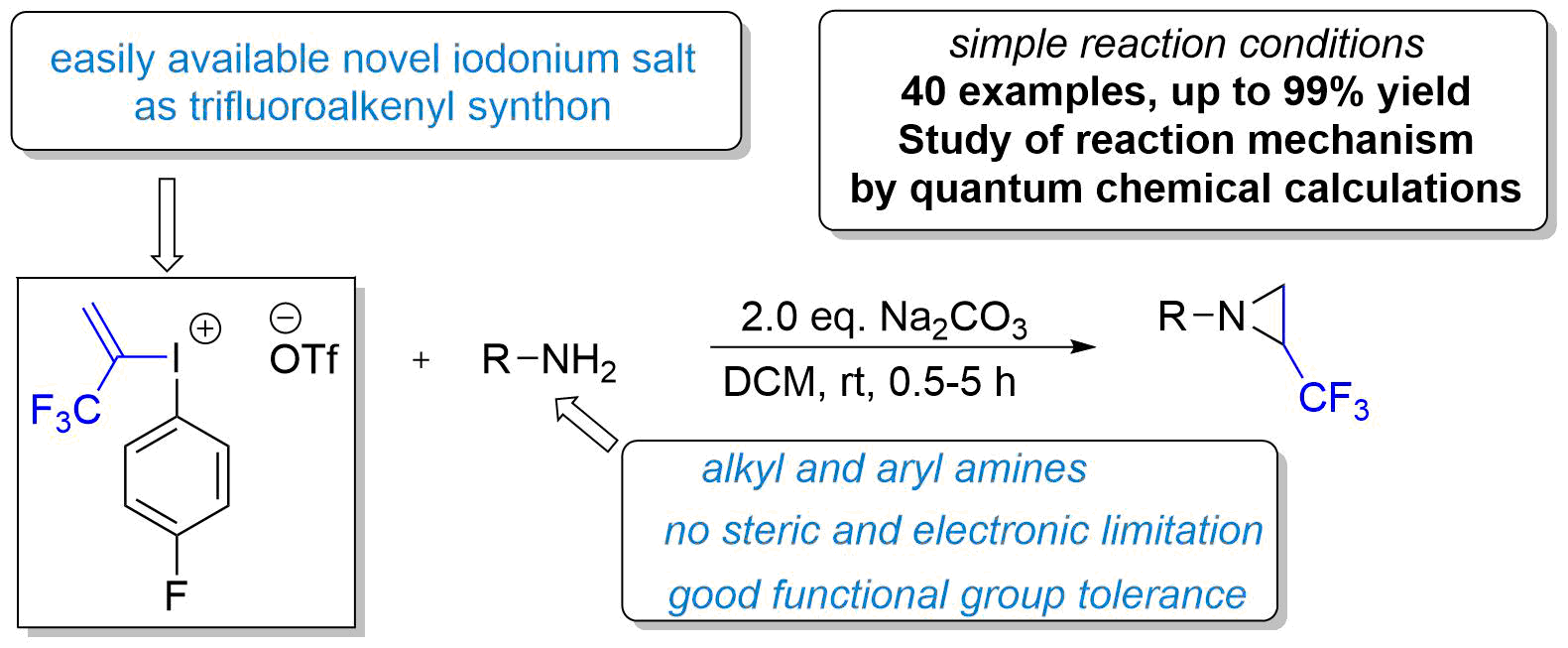
Our paper entitled "Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring" was highlighted by OPR&D! The highlights are selected by a group of industrial chemists as a service to the readership who has a strong interest in practical chemistry that addresses and solves industrial questions.
The efficient construction of small heterocyclic fluorinated building blocks continues to be a major topic of interest for the synthetic community. Novák and co-workers at Eötvös University in Budapest described a new methodology for the synthesis of trifluoromethylated aziridines that expands for the first time the scope of substrates to nonaromatic amines ( Angew. Chem., Int. Ed. 2018, 57, 6643). For that purpose, the authors designed and synthesized trifluoropropenyl iodonium salts as new C2–CF3 synthons. Under the best conditions, dichloromethane as the solvent with 2 equiv of sodium carbonate as the base, an array of substituted alkylamines as well as (hetero)arylamines were converted into trifluoromethylated aziridines in moderate to high yields. An impressive number of functional groups, including alcohols and unprotected anilines, are well-tolerated. DFT calculations were performed to provide insight into the mechanism of the reaction.
Wenyi Zhao, Sylvain Guizzetti, James A. Schwindeman, David S. B. Daniels, Carlos A. Guerrero, David Philip Day, John Knight, Org. Process Res. Dev. 2018, 22, 907-917. DOI: 10.1021/acs.oprd.8b00238 | [Full Text Link]

Two New Publications on Pd Catalysis
This June started with two new papers related to palladium cross-coupling reactions.
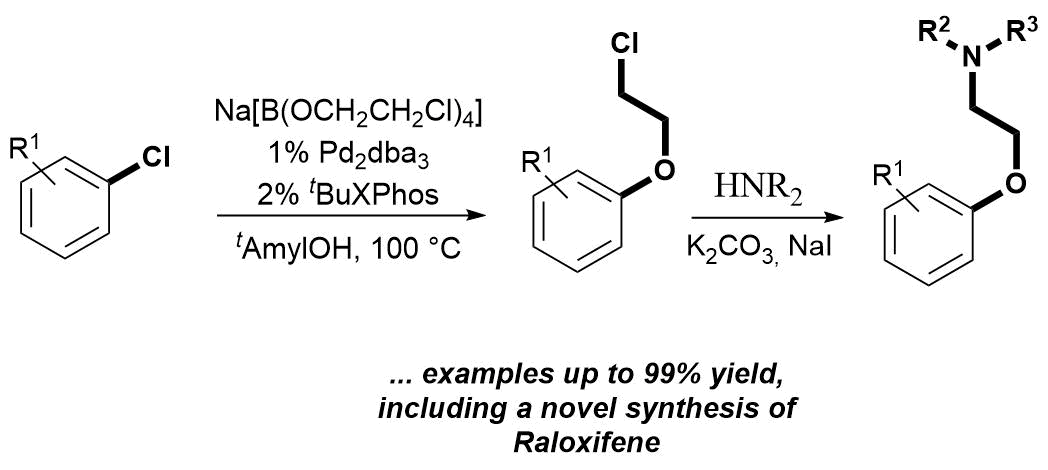
First, we developed palladium-catalyzed functionalization of aryl- and heteroaryl chlorides with sodium tetrakis-(2-chloroethoxy)-borate salt, which is orthogonal to the simple nucleophilic replacement of the chloro function of the ethylene linker. Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker. Subsequent amine substitution of the alkyl halide affords 2-aminoethoxy arenes. The paper entitled as "Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: an Orthogonal Functionalization of Chloroethoxy Linker" and published in Organic & Biomolecular Chemistry (RSC). For further reading, please, Click here.
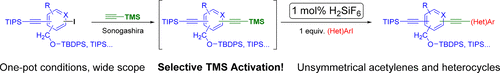
Second, we disclose a novel catalytic method for the selective deprotection of trimethylsilylacetylenes in Sonogashira reaction. The reagent hexafluorosilicic acid, an inexpensive nontoxic compound, was used to promote the selective desilylation. This paper published by ACS in The Journal of Organic Chemistry as "Catalytic Activation of Trimethylsilylacetylenes: A One-Pot Route to Unsymmetrical Acetylenes and Heterocycles". For further reading, please, Click here.
Synthesis of CF3-Aziridines Published in Angewandte Chemie
A new route has been discovered to obtain CF3 substituated N-aryl, alkyl and tosylaziridine products. With the utilization of a newly designed, bench stable but highly reactive hypervalent alkenyl iodonium species, the three membered heterocyclic ring can be constructed from simple amines without structural limitation with high efficiency under mild conditions in the absence of transition metal catalysts. The paper entitled as "Design of Trifluoroalkenyliodonium salts for Hypervalency Aided Alkenylation‐Cyclization Strategy: Metal‐free Construction of Aziridine Ring" and published in Angewandte Chemie International Edition (Wiley). For further reading, please, Click here.
First hit in 2018! Aromatic C-H Alkylation by Sulfonium Salts
C−C bond-forming reactions via aromatic C−H bond activation processes have been studied in this paper. C−H activation based direct alkylations by alkyl group transfer are less explored in this field. However, the introduction of alkyl groups into aromatic systems, especially the ubiquitous methyl group in molecular scaffolds, could have beneficial effects on biological properties. This paper describes "Sulfonium Salts as Alkylating Agents for Palladium-Catalyzed Direct Ortho Alkylation of Anilides and Aromatic Ureas" presented by Dániel Simkó et. al. in Organic Letters (ACS). For further reading, please, Click here.
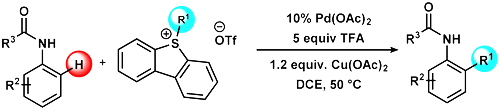
OPR&D Highlights
Our new publication entitled "Palladium Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of Trifluoro Analog of Sildenafil" had been picked up by the Highlights of OPR&D! The highlights are selected by a group of industrial chemists as a service to the readership who has a strong interest in practical chemistry that addresses and solves industrial questions.These trifluoroethoxylated compounds are both very useful and not always that easy to produce.
Wenyi Zhao, Dongbo Zhao, Sylvain Guizzetti, James A. Schwindeman, David S. B. Daniels, Carlos A. Guerrero, Arjun Raghuraman, John Knight, Org. Process Res. Dev. 2017, 21, 1873-1883. DOI: 10.1021/acs.oprd.7b00371 | [Full Text Link]

