September starts with two new publications
We are pleased to share our new papers.
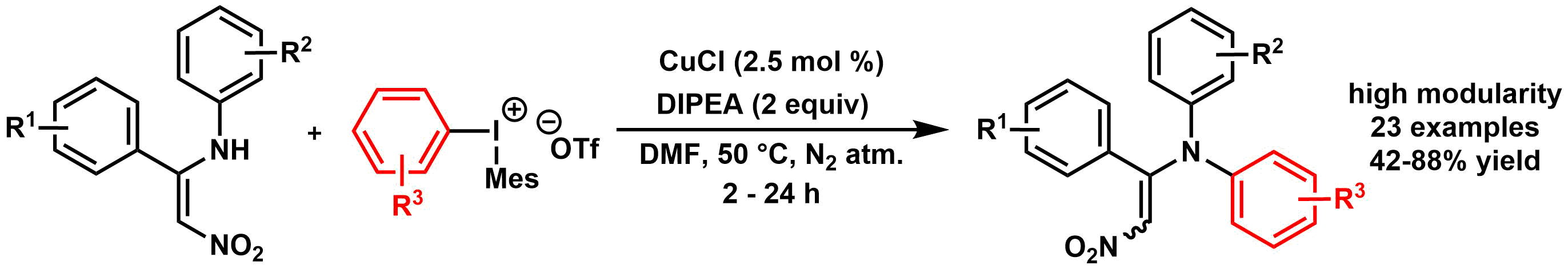
Aradi K. and her co-workers presented a mild N-arylation of nitroenamine derivatives utilizing diaryliodonium triflates and copper(I) chloride as a catalyst. For further reading, please, click here.
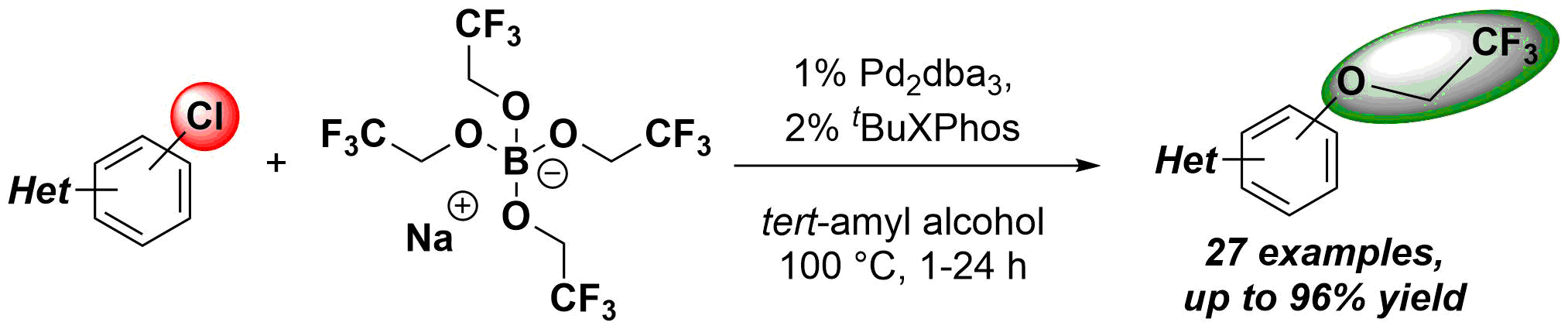
Pethő B. and his co-workers developed a synthetic method for 2,2,2-trifluoroethoxylation of aromatic and heteroaromatic chlorides utilizing borate salt. Under palladium catalyzed reaction conditions an additional analog of Sildenafil was synthetized also. The paper was published in Chemistry - A European Journal (Wiley).For further reading, please, click here.
New method for sequential coupling and deprotection of silyl alkynes
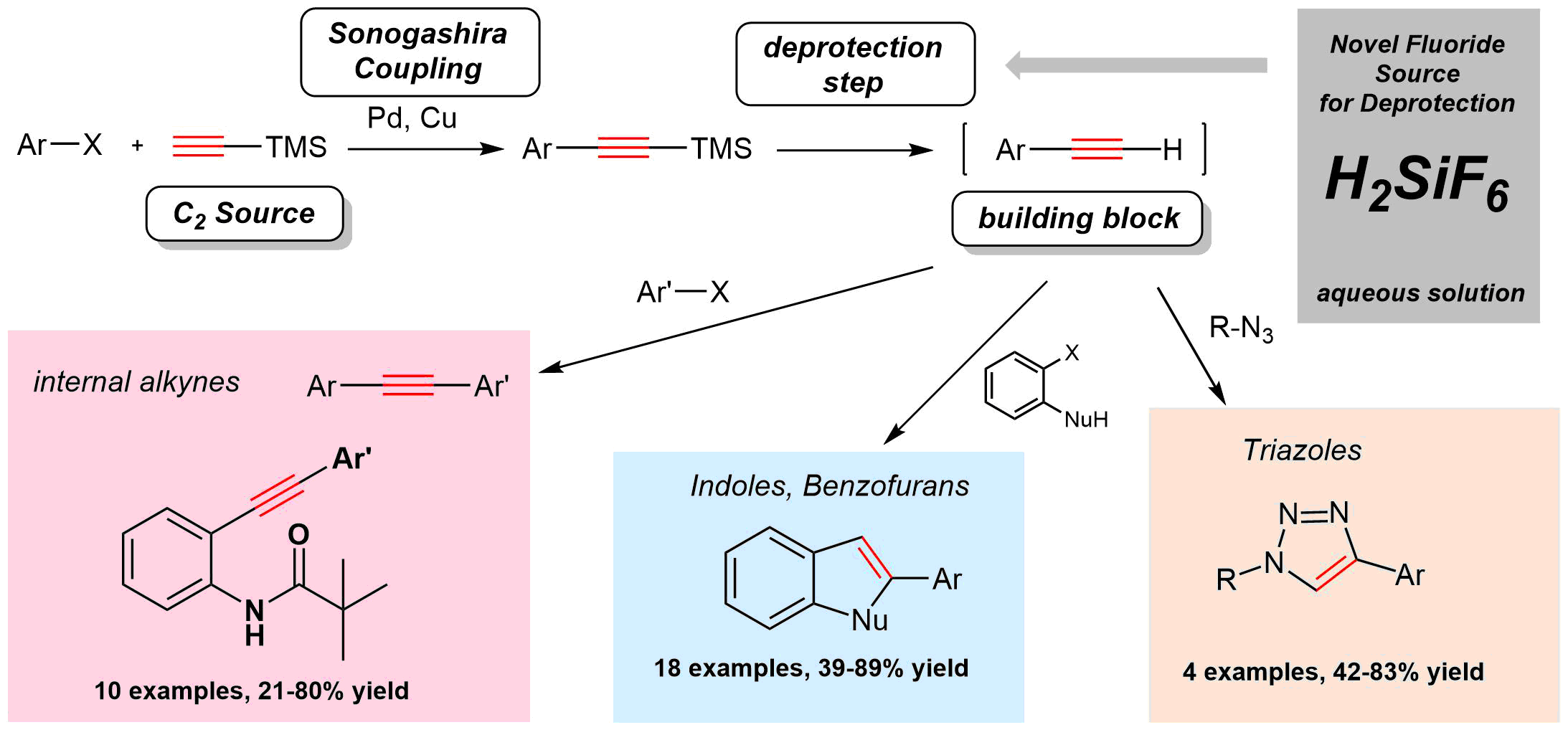
Our group was presented a new syhtetic method for the desilylation of silylacetylenes via aquaous solution of hexafluorosilicic acid. The applicability of the novel, cheap and environmentally friendly reagent was demonstrated in the sequential coupling of aryl halides and ethynyltrimethylsilane to afford internal acetylenes, benzofurans, and triazoles in one-pot reactions. This paper has been published in Synthesis (Thieme) and entitled as "Hexafluorosilicic Acid as a Novel Reagent for the Desilylation of Silylacetylenes: Application in Sequential Sonogashira Coupling and Click Reaction". For further reading, please, click here.
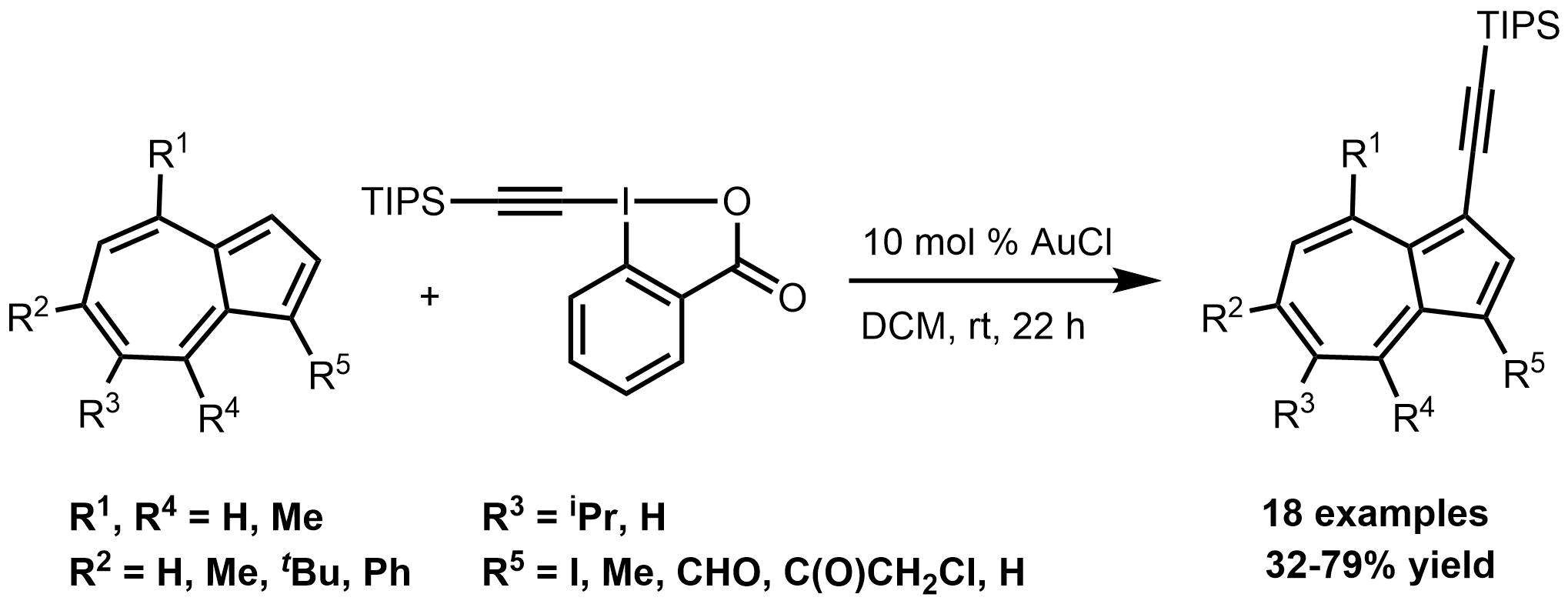
Synthesis of Azulenes in Organic Letters
Our new paper has been published in topic of azulene chemistry, in Organic Letters (ACS)! This paper entitled as "Gold-Catalyzed Direct Alkynylation of Azulenes" and contains 18 examples of novel TIPS-acetilene-azulenes. For further reading please, Click Here.
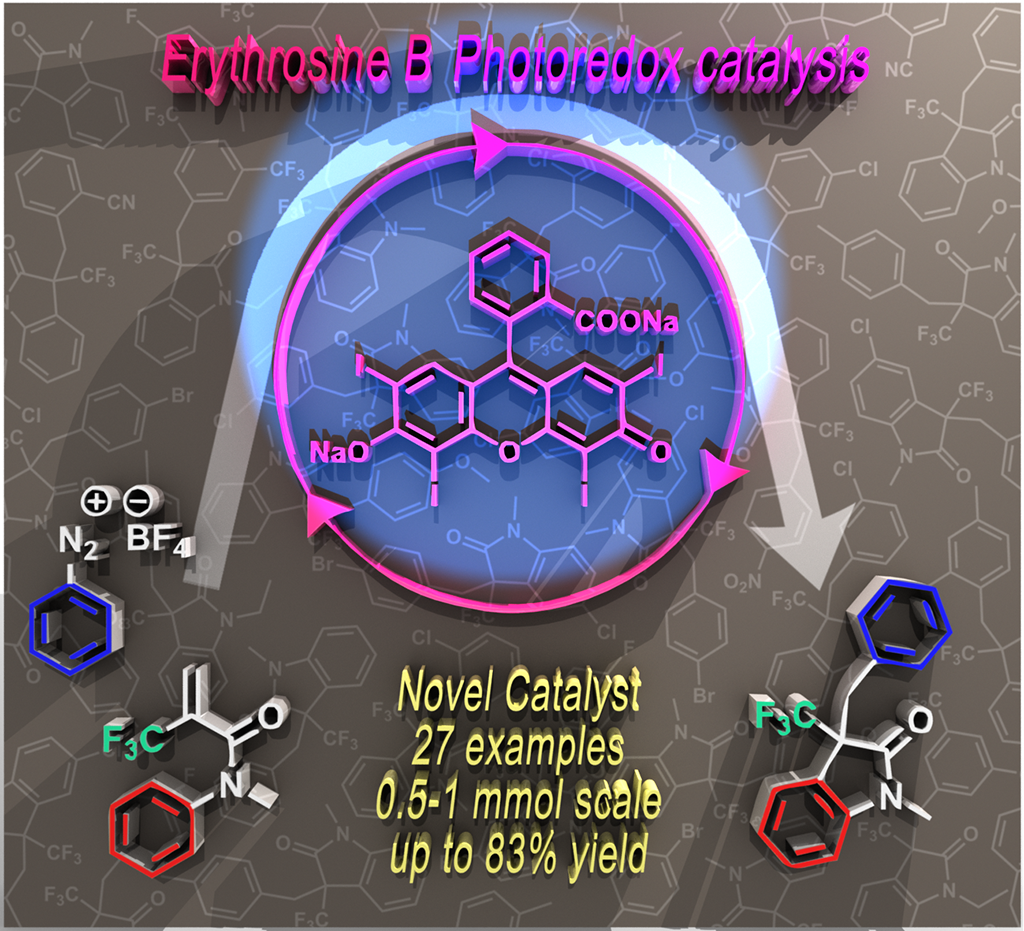
Our new paper will be published in Eur. J. Org. Chem. special issue
The groups last publication has been accepted for publication in European Journal of Organic Chemistry (Wiley). This project was entitled as "Erythrosine B catalyzed visible-light photoredox arylation-cyclization of N-alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(trifluoromethyl)indolin-2-one derivatives" and presented by Zsombor Gonda, Ferenc Béke, Orsolya Tischler, Milán Petró, Zoltán Novák and Balázs Tóth. In this work, the novel erythrosine B organic sensitizer dye was utilized as photocatalyst to cyclize the trifluoroacrylamides. You can read this paper already in Accepted Articles section at Wiley. For further reading please, Click Here.
New Paper on Trifluoroethylation of Urea Derivatives
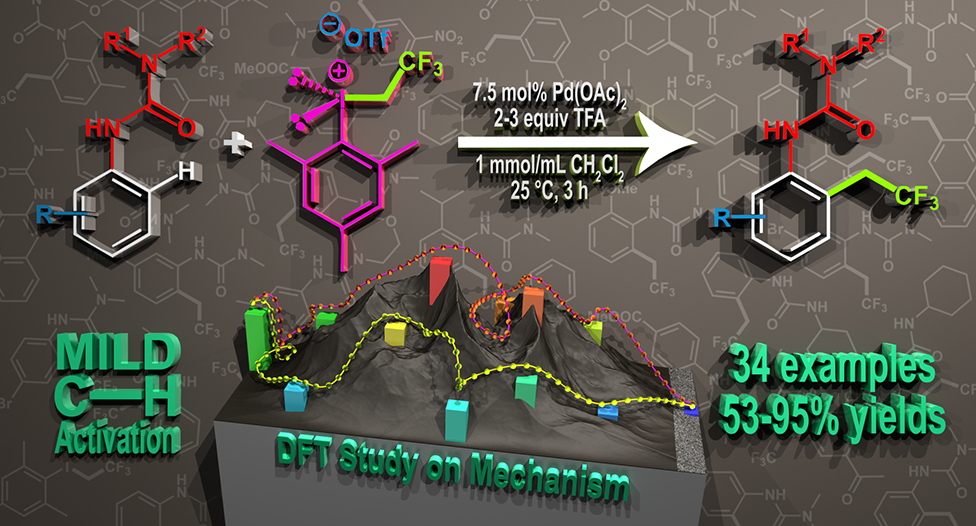
Our new publication was accepted in Advanced Synthesis & Catalysis (Wiley). This paper is about "Direct ortho-Trifluoroethylation of Aromatic Ureas by Palladium Catalyzed C-H activation: A Missing Piece of Aromatic Substitutions" and released yesterday (15.12.2016). The authors, Szabolcs Kovács, Balázs L. Tóth, Gábor Borsik, Tamás Bihari, Nóra V. May, András Stirling and Zoltán Novák reported a easily feasible method for trifluoroethylation, which was unprecedented transformation considering the direct alkylation of ureas.
Abstract: Development of direct late-stage installation of alkyl groups into aromatic systems is an important and challenging task of current organic chemistry. In spite of the existing functionalization methods in organic chemistry for the substitution reactions on aromatic systems, the direct alkylation of aromatic ureas is unknown. Herein, as a first example we report a novel palladium catalyzed fluoroalkylation process by C−H activation for the access of ortho trifluoroethylated aromatic ureas. The application of novel, highly active trifluoroethyl(mesityl)iodonium salt enables the efficient introduction of the trifluoroethyl group at 25 °C in 3 hours in high yields (up to 95%) with good functional group tolerance. DFT calculations have revealed a rate determining oxidative alkyl-group transfer preceded by an unexpected C−H activation route on the Pd center during the catalytic cycle, where the deprotonation is assisted by an external triflate anion. For further reading please, Click Here. DOI: 10.1002/adsc.201601136